Cyheptamide
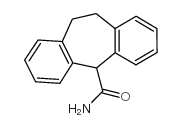
Cyheptamide structure
|
Common Name | Cyheptamide | ||
|---|---|---|---|---|
| CAS Number | 7199-29-3 | Molecular Weight | 237.29600 | |
| Density | 1.173 g/cm3 | Boiling Point | 438.8ºC at 760 mmHg | |
| Molecular Formula | C16H15NO | Melting Point | 193-194ºC | |
| MSDS | Chinese USA | Flash Point | 219.2ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
A simple, efficient, and sensitive method for simultaneous detection of anti-HIV drugs atazanavir, ritonavir, and tenofovir by use of liquid chromatography-tandem mass spectrometry.
Antimicrob. Agents Chemother. 59 , 6682-8, (2015) In the treatment of HIV infection, a combination of anti-HIV drugs is commonly used in highly active antiretroviral therapy (HAART). One such combination recommended for clinical therapy consists of the two HIV protease inhibitors atazanavir and ritonavir and... |
|
|
Evaluation of atazanavir and darunavir interactions with lipids for developing pH-responsive anti-HIV drug combination nanoparticles.
J. Pharm. Sci. 103(8) , 2520-9, (2014) We evaluated two human immunodeficiency virus (HIV) protease inhibitors, atazanavir (ATV) and darunavir (DRV), for pH-dependent solubility, lipid binding, and drug release from lipid nanoparticles (LNPs). Both ATV and DRV incorporated into LNPs composed of pe... |
|
|
Cyheptamide and 3-hydroxy-3-phenacyloxindole: structural similarity to diphenylhydantoin as the basis for anticonvulsant activity.
J. Med. Chem. 27(5) , 649-54, (1984) The molecular structures of cyheptamide and 3-hydroxy-3- phenacyloxindole were determined by X-ray diffraction methods. The amide group in both compounds exhibits delocalization of the pi-electrons over the three atoms (N, C, and O), while the bond linking th... |
|
|
Anticonvulsant activities and brain concentrations of cyheptamide and carbamazepine.
Proc. West. Pharmacol. Soc. 23 , 75-9, (1980)
|
|
|
Comparison of anticonvulsant potencies of cyheptamide, carbamazepine, and phenytoin.
J. Pharm. Sci. 70(6) , 618-20, (1981) Carbamazepine and cyheptamide have certain stereochemical features in common with phenytoin; when superimposed, two bulky hydrophobic groups in each permit the approximate apposition of two electron donor atoms. The anticonvulsant activity of each compound wa... |
|
|
A sensitive capillary GC-MS method for analysis of topiramate from plasma obtained from single-dose studies.
Biomed. Chromatogr. 26(9) , 1071-6, (2012) Topiramate (Topamax®) is an antiepileptic medication used as adjunctive and monotherapy in patients with epilepsy and for migraine prophylaxis. A GC-MS assay was developed that was capable of detecting topiramate plasma concentrations following a single recta... |
|
|
[Determination of plasma lidocaine by gas-liquid chromatography].
Rev. Med. Chil. 115(7) , 661-4, (1987)
|