nevirapine
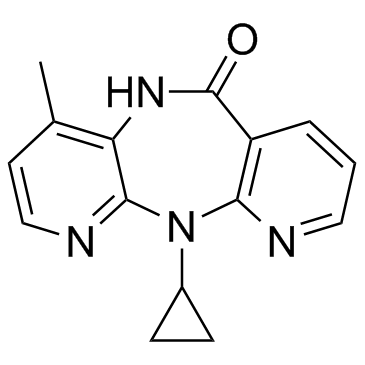
nevirapine structure
|
Common Name | nevirapine | ||
|---|---|---|---|---|
| CAS Number | 129618-40-2 | Molecular Weight | 266.298 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 415.4±45.0 °C at 760 mmHg | |
| Molecular Formula | C15H14N4O | Melting Point | 247°C | |
| MSDS | Chinese USA | Flash Point | 205.0±28.7 °C | |
|
Livolin ameliorates elevations in alanine transaminase in HIV infected patients commencing highly active antiretroviral therapy.
Afr. J. Med. Med. Sci. 41(4) , 417-22, (2012) HAART associated hepatoxicity is an important cause of poor adherence to therapy in HIV infected persons. An initial manifestation is elevation in the level ofAlanine Transaminase (ALT) in blood. We sought to evaluate the protective effects of Livolin, a phos... |
|
|
Nucleoside reverse transcriptase inhibitor resistance mutations associated with first-line stavudine-containing antiretroviral therapy: programmatic implications for countries phasing out stavudine.
J. Infect. Dis. 207 Suppl 2 , S70-7, (2013) The World Health Organization Antiretroviral Treatment Guidelines recommend phasing-out stavudine because of its risk of long-term toxicity. There are two mutational pathways of stavudine resistance with different implications for zidovudine and tenofovir cro... |
|
|
HIV testing in pregnancy.
Sex. Transm. Infect. 90(8) , 640, (2014)
|
|
|
Uptake of prevention of mother-to-child-transmission using Option B+ in northern rural Malawi: a retrospective cohort study.
Sex. Transm. Infect. 90(4) , 309-14, (2014) To identify points of dropout on the pathway from offering HIV testing to maintenance on antiretroviral therapy (ART), following the introduction of the Option B+ policy for pregnant women in Malawi (lifelong ART for HIV-positive mothers and 6 weeks nevirapin... |
|
|
Effect of highly active antiretroviral treatment (HAART) during pregnancy on pregnancy outcomes: experiences from a PMTCT program in western India.
AIDS Patient Care STDS 27(3) , 163-70, (2013) Previous research regarding the effect of highly active antiretroviral treatment (HAART) on pregnancy outcomes shows conflicting results and is predominantly situated in developed countries. Recently, HAART is rapidly being scaled up in developing countries f... |
|
|
Toxicology and carcinogenesis studies of mixtures of 3'-azido-3'-deoxythymidine (AZT), lamivudine (3TC), nevirapine (NVP), and nelfinavir mesylate (NFV) (Cas Nos. 30516-87-1, 134678-17-4, 129618-40-2, 159989-65-8) in B6C3F1 Mice (transplacental exposure studies).
Natl. Toxicol. Program Tech. Rep. Ser. (569) , 1-212, (2013) Antiretroviral drugs are used to treat patients positive for the human immunovirus HIV-1, and increasingly treatments include a combination of such drugs. The noninfected children of women who are pregnant and receiving such treatment may also be exposed to t... |
|
|
Nevirapine inhibits the anti-HIV activity of CD8+ cells.
J. Acquir. Immune Defic. Syndr. 63(2) , 184-8, (2013) Antiretroviral therapy (ART) significantly reduced the CD8 cell noncytotoxic anti-HIV response in 12 HIV-1-infected subjects (P < 0.0001). In separate experiments, CD8(+) cells from long-term survivors were cocultured with HIV-infected CD4(+) cells using vary... |
|
|
High throughput LC-MS/MS method for simultaneous determination of zidovudine, lamivudine and nevirapine in human plasma.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 921-922 , 9-14, (2013) A selective and sensitive high performance liquid chromatography-tandem mass spectrometry method has been developed and validated for simultaneous determination of zidovudine (ZDV), lamivudine (3TC) and nevirapine (NVP) in human plasma. After Solid phase extr... |
|
|
Short communication: effect of short-course antenatal zidovudine and single-dose nevirapine on the BED capture enzyme immunoassay levels in HIV type 1 subtype C infection.
AIDS Res. Hum. Retroviruses 29(6) , 901-6, (2013) Cross-sectional prevalence studies based on immunoassays that discriminate between recent and long-term infections, such as the BED assay, have been widely used to estimate HIV incidence. However, individuals receiving highly active antiretroviral therapy ten... |
|
|
Baseline CD4 cell counts and outcomes among adult treatment naive HIV patients after taking fixed dose combination GPO-VIR-S and GPO-VIR-Z in Thailand.
Southeast Asian J. Trop. Med. Public Health 44(2) , 232-43, (2013) A retrospective study was conducted by reviewing 459 medical records of adult treatment naive HIV patients who received a fixed dose combination of GPO-VIR-S (stavudine, lamivudine and nevirapine) or GPO-VIR-Z (zidovudine, lamivudine and nevirapine) at Ramath... |