1-Bromo-2-nitrobenzene
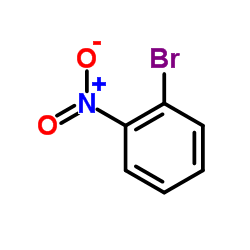
1-Bromo-2-nitrobenzene structure
|
Common Name | 1-Bromo-2-nitrobenzene | ||
|---|---|---|---|---|
| CAS Number | 577-19-5 | Molecular Weight | 202.005 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 261.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C6H4BrNO2 | Melting Point | 40-42 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 87.8±19.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Organo-palladium(II) complexes bearing unsymmetrical N,N,N-pincer ligands: synthesis, structures and oxidatively induced coupling reactions.
Dalton Trans. 44(16) , 7230-41, (2015) The 2-(2′-aniline)-6-imine-pyridines, 2-(C6H4-2′-NH2)-6-(CMe=NAr)C5H3N (Ar = 4-i-PrC6H4 (HL1a), 2,6-i-Pr2C6H3 (HL1b)), have been synthesised via sequential Stille cross-coupling, deprotection and condensation steps from 6-tributylstannyl-2-(2-methyl-1,3-dioxo... |
|
|
Synthesis of quinolines, 2-quinolones, phenanthridines, and 6(5h)-phenanthridinones via palladium[0]-mediated Ullmann cross-coupling of 1-bromo-2-nitroarenes with beta-halo-enals, -enones, or -esters.
Org. Lett. 6(16) , 2741-4, (2004) Palladium[0]-mediated Ullmann cross-coupling of 1-bromo-2-nitrobenzene (1 R = H) and its derivatives with a range of beta-halo-enals, -enones, or -esters readily affords the corresponding beta-aryl derivatives, which are converted into the corresponding quino... |
|
|
New protocols for the synthesis of 3, 4-annulated and 4-substituted quinolines from ß-bromo-a, ß-unsaturated aldehydes and 1-bromo-2-nitrobenzene or 2-bromoacetanilide. Some S, et al.
Tetrahedron Lett. 48(20) , 3609-12, (2007)
|
|
|
4-Hydroxy-2'-Nitrodiphenyl Ether Analogues as Novel Tyrosinase Inhibitors. Sapkota K, et al.
Bull. Korean Chem. Soc. 31(5) , 1319, (2010)
|