2-Chlorobenzaldehyde
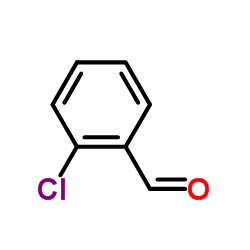
2-Chlorobenzaldehyde structure
|
Common Name | 2-Chlorobenzaldehyde | ||
|---|---|---|---|---|
| CAS Number | 89-98-5 | Molecular Weight | 140.567 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 211.9±0.0 °C at 760 mmHg | |
| Molecular Formula | C7H5ClO | Melting Point | 9-11 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 87.8±0.0 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Acetalization allows the photoheterolysis of the Ar-Cl bond in chlorobenzaldehydes and chloroacetophenones.
J. Org. Chem. 77(20) , 9094-101, (2012) The nonaccessibility of phenyl cations by irradiation of electron-poor aryl chlorides was circumvented by transforming the carbonyl group of aromatic ketones or aldehydes into the corresponding 1,3-dioxolanes and the carboxyl group of benzoate ester into an o... |
|
|
Experimental and theoretical studies of the products of addition-elimination reactions between benzil dihydrazone and three isomeric chlorobenzaldehydes.
Acta Crystallogr. C Struct. Chem. 71 , 554-63, (2015) A series of mono- and di-Schiff bases formed between benzil dihydrazone {BDH; systematic name: (1Z)-[(2E)-2-hydrazinylidene-1,2-diphenylethylidene]hydrazine} and three isomeric chlorobenzaldehydes were designed and synthesized to be used as model compounds to... |
|
|
β-Olefination of 2-alkynoates leading to trisubstituted 1,3-dienes.
Org. Lett. 13(13) , 3418-21, (2011) A phosphine-mediated olefination of 2-alkynoates with aldehydes forming 1,3-dienes with high E-selectivity and up to 88% yield is described. Reaction conditions are optimized and reactions are demonstrated for various aryl, alkyl, and alkenyl aldehydes and fo... |
|
|
Vibrational spectra of partially oriented molecules having two conformers in nematic and isotropic solutions: furfural and 2-chlorobenzaldehyde.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 61(7) , 1661-70, (2005) Vibrational analysis of the two conformers of furfural and 2-chlorobenzaldehyde has been carried out on the basis of their IR and Raman spectra measured in isotropic and anisotropic (nematic liquid crystalline) solvent. The average orientation of the individu... |
|
|
Analysis of the aneuploidy inducing capacity of 2-chlorobenzylidene malonitrile (CS) and metabolites in V79 Chinese hamster cells.
Mutagenesis 6(4) , 303-5, (1991) The aneuploidy inducing capacity of 2-chlorobenzylidene malonitrile (CS), a chemical used as a riot control agent, and its hydrolysis products o-chlorobenzaldehyde and malonitrile was studied at various exposure conditions in V79 Chinese hamster cells. Chromo... |
|
|
Synthesis of ionones and carvone analogues: olfactory properties and preliminary toxicity assays.
Eur. J. Med. Chem. 35(9) , 797-803, (2000) Vilsmeier reagents react with alpha/beta-ionones and carvone to produce aldehydes 7-11 in a one-step procedure. The indene derivative 11, which came from the double iminoalkylation of carvone and ring closure with the elimination of dimethylamine, was practic... |
|
|
Percutaneous absorption of 14C-labelled 2-chlorobenzaldehyde in rats. Metabolism and toxicokinetics.
Eur. J. Drug Metab. Pharmacokinet. 13(4) , 231-40, (1988) 2-Chlorobenzaldehyde might be produced when a moist skin is exposed to the riot control agent CS. CS-hydrolysis to 2-chlorobenzaldehyde and malononitrile occurs both in vitro and in vivo. No quantitative data have thus far been reported with respect to the pe... |
|
|
Anisotropy shift and Raman bandwidth studies in carbonyl containing molecule o-chlorobenzaldehyde: role of repulsive forces.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 62(4-5) , 972-9, (2005) The analysis of Raman anisotropy shift as a function of solvent concentration shows the weakening of pair interaction of the molecules due to the influence of solvent-induced perturbations. The present study deals with the effect of dielectric constant of the... |
|
|
Automated generation of a dihydropyrimidine compound library using microwave-assisted processing.
Nat. Protoc. 2(7) , 1713-21, (2007) Here we report the generation of a small focused library of 12 diversely functionalized dihydropyrimidine (DHPM) derivatives via one-pot three-component Biginelli cyclocondensation of beta-ketoesters, aldehydes and (thio)ureas. By applying controlled microwav... |
|
|
Enantioselective alkynylation of aromatic aldehydes catalyzed by new chiral amino alcohol-based ligands. Lu G, et al.
Tetrahedron Asymmetry 12(15) , 2147-2152, (2001)
|