3-ACETYLPHENOL
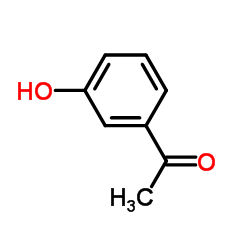
3-ACETYLPHENOL structure
|
Common Name | 3-ACETYLPHENOL | ||
|---|---|---|---|---|
| CAS Number | 121-71-1 | Molecular Weight | 136.148 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 277.0±23.0 °C at 760 mmHg | |
| Molecular Formula | C8H8O2 | Melting Point | 90-95 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 115.8±15.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Convenient QSAR model for predicting the complexation of structurally diverse compounds with β-cyclodextrins
Bioorg. Med. Chem. 17 , 896-904, (2009) This paper reports a QSAR study for predicting the complexation of a large and heterogeneous variety of substances (233 organic compounds) with beta-cyclodextrins (beta-CDs). Several different theoretical molecular descriptors, calculated solely from the mole... |
|
|
Virtual screening identification of nonfolate compounds, including a CNS drug, as antiparasitic agents inhibiting pteridine reductase.
J. Med. Chem. 54 , 211-221, (2011) Folate analogue inhibitors of Leishmania major pteridine reductase (PTR1) are potential antiparasitic drug candidates for combined therapy with dihydrofolate reductase (DHFR) inhibitors. To identify new molecules with specificity for PTR1, we carried out a vi... |
|
|
Synthesis of new UV-B light absorbents: (Acetylphenyl)glycosides with antioxidant activities.
Bioorg. Med. Chem. Lett. 18 , 3582-4, (2008) m-Acetylphenyl-beta-d-glucopyranosides and m-acetylphenyl-alpha/beta-d-mannopyranosides were synthesized by the Koenigs-Knorr, Mitsunobu, and Helferich reactions as key glycosylation reactions, respectively. Their spectroscopic properties and antioxidative ac... |
|
|
Chemoenzymatic synthesis of rivastigmine via dynamic kinetic resolution as a key step.
J. Org. Chem. 75(9) , 3105-8, (2010) A practical and efficient procedure for the synthesis of rivastigmine was developed. This procedure includes dynamic kinetic resolution using a polymer-bound ruthenium complex and a lipase in combination as a key step. Enantiopure (-)-rivastigmine was obtaine... |
|
|
Controlling dissociation of alkyl phenyl ketone radical cations in the strong-field regime through hydroxyl substitution position.
J. Phys. Chem. A , (2014) The hydroxy-substituted alkyl phenyl ketones 2'-, 3'- and 4'- (ortho, meta, and para) hydroxyacetophenone were excited in the strong-field regime with wavelengths ranging from 1200-1500 nm to produce the respective radical cations. For 2'- and 3'-hydroxyaceto... |
|
|
Synthesis of polyester dendrimers and dendrons starting from Michael reaction of acrylates with 3-hydroxyacetophenone. Hirayama Y, et a
Tetrahedron Lett. 46(6) , 1027-30, (2005)
|