Hydroxylamine-O-Sulfonic Acid
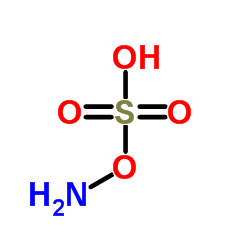
Hydroxylamine-O-Sulfonic Acid structure
|
Common Name | Hydroxylamine-O-Sulfonic Acid | ||
|---|---|---|---|---|
| CAS Number | 2950-43-8 | Molecular Weight | 113.093 | |
| Density | 2.0±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | H3NO4S | Melting Point | 210 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Elusive metal-free primary amination of arylboronic acids: synthetic studies and mechanism by density functional theory.
J. Am. Chem. Soc. 134(44) , 18253-6, (2012) Herein, we disclose the first metal-free synthesis of primary aromatic amines from arylboronic acids, a reaction that has eluded synthetic chemists for decades. This remarkable transformation affords structurally diverse primary arylamines in good chemical yi... |
|
|
Co-immobilization of semaphorin3A and nerve growth factor to guide and pattern axons.
Acta Biomater. 28 , 33-44, (2015) Immobilization of axon guidance cues offers a powerful tissue regenerative strategy to control the presentation and spatial location of these biomolecules. We use our previously developed immobilization strategy to specifically tether recombinant biotinylated... |
|
|
Aminations of guanosine and deoxyguanosine with hydroxylamine-O-sulfonic acid and 2,4-dinitrophenoxyamine. Dependence on the reaction medium.
Nucleic Acids Symp. Ser. (17) , 145-8, (1986) Amination of guanosine (Guo) with 2,4-dinitrophenoxyamine in aqueous DMF gave 7-amino-Guo, which was readily converted to 8,5'-O-cyclo-Guo, and 8-hydroxy-Guo. Deoxyguanosine (dG) gave only deglycosylated 7-amino-G under the same reaction condition. Aminations... |
|
|
Mechanism of metal-mediated DNA damage induced by metabolites of carcinogenic 2-nitropropane.
Mutat. Res. 479(1-2) , 101-11, (2001) 2-Nitropropane (2-NP), a widely used industrial solvent, is carcinogenic to rats. To clarify the mechanism of carcinogenesis by 2-NP, we investigated DNA damage by 2-NP metabolites, N-isopropylhydroxylamine (IPHA) and hydroxylamine-O-sulfonic acid (HAS), usin... |
|
|
The reaction of organoboranes with chloramine and with hydroxylamine-O-sulfonic acid. A convenient synthesis of amines from olefins via hydroboration. Brown HC, et al.
J. Am. Chem. Soc. 86(17) , 3565-3566, (1964)
|
|
|
Hydroxylamine-O-sulfonic Acid. Erdik E and Saczewski J.
e-EROS Encyclopedia of Reagents for Organic Synthesis. , (2013)
|
|
|
Synthesis and molecular structure of (Z)-1H-purin-6-ylideneaminooxysulfonic acid: a possible secondary metabolite of adenine. Saczewski J and Gdaniec M.
Heterocycl. Comm. 18(3) , 109-112, (2012)
|
|
|
Amination of tetrazoles with hydroxylamine-O-sulfonic acid: 1-and 2-aminotetrazoles. Raap R.
Can. J. Chem. 47(19) , 3677-3681, (1969)
|
|
|
N-Trinitroethylamino functionalization of nitroimidazoles: a new strategy for high performance energetic materials. Yin P, et al.
J. Mater. Chem. A 1(25) , 7500-7510, (2013)
|
|
|
A New Strategy for the Preparation of N-Aminopiperidine Using Hydroxylamine-O-Sulfonic Acid: Synthesis, Kinetic Modelling, Phase Equilibria, Extraction and Processes. Labarthe E, et al.
Adv. Chem. Eng. Sci. 3(2) , (2013)
|