2-Aminobenzimidazole
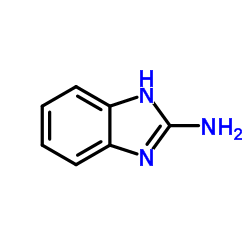
2-Aminobenzimidazole structure
|
Common Name | 2-Aminobenzimidazole | ||
|---|---|---|---|---|
| CAS Number | 934-32-7 | Molecular Weight | 133.151 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 368.9±25.0 °C at 760 mmHg | |
| Molecular Formula | C7H7N3 | Melting Point | 226-230 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 204.6±10.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Aggregation of 2-aminobenzimidazole--a combined experimental and theoretical investigation.
ChemPhysChem 12(9) , 1747-55, (2011) An investigation of 2-aminobenzimidazole was carried out by calculations at HF, MP2, and DFT levels of theory and also by UV and IR spectroscopy. The quantum chemical calculations predict a full shift of the equilibrium towards the amino form, but the absorpt... |
|
|
Brain-penetrating 2-aminobenzimidazole H(1)-antihistamines for the treatment of insomnia.
Bioorg. Med. Chem. Lett. 19(15) , 4380-4, (2009) The benzimidazole core of the selective non-brain-penetrating H(1)-antihistamine mizolastine was used to identify a series of brain-penetrating H(1)-antihistamines for the potential treatment of insomnia. Using cassette PK studies, brain-penetrating H(1)-anti... |
|
|
Chemical biology strategy reveals pathway-selective inhibitor of NF-kappaB activation induced by protein kinase C.
ACS Chem. Biol. 5 , 287-299, (2010) Dysregulation of NF-kappaB activity contributes to many autoimmune and inflammatory diseases. At least nine pathways for NF-kappaB activation have been identified, most of which converge on the IkappaB kinases (IKKs). Although IKKs represent logical targets f... |
|
|
Identification of 2-aminobenzimidazoles as potent melanin-concentrating hormone 1-receptor (MCH1R) antagonists.
Bioorg. Med. Chem. Lett. 19(13) , 3568-72, (2009) A series of 2-aminobenzimidazole-based MCH1R antagonists was identified by core replacement of the aminoquinoline lead 1. Subsequent modification of the 2- and 5-positions led to improvement in potency and intrinsic clearance. Compound 25 exhibited good plasm... |
|
|
Synthesis and QSAR studies of novel 1-substituted-2-aminobenzimidazoles derivatives.
Eur. J. Med. Chem. 41(9) , 1080-3, (2006) A series of novel 1-substituted-2-aminobenzimidazole derivatives were synthesized. The structures of the synthesized compounds were confirmed by 1H-NMR spectra and by elemental analysis. Acute toxicities of these compounds were detected on mice via toxicity (... |
|
|
Experimental and theoretical (DFT) characterization of the excited states and N-centered radical species derived from 2-aminobenzimidazole, the core substructure of a family of bioactive compounds.
J. Phys. Chem. B 114(19) , 6608-13, (2010) Fluorescence spectroscopy, laser flash photolysis (LFP), and density functional theory (DFT) calculations have been performed to understand the photobehavior of 2-aminobenzimidazole (1). The emission lifetime and quantum yield are solvent-dependent. Direct LF... |
|
|
Simultaneous analysis of thiabendazole, carbendazim and 2-aminobenzimidazole in concentrated fruit juices by liquid chromatography after a single mix-mode solid-phase extraction cleanup.
J. Environ. Sci. Health B 44(6) , 591-7, (2009) A method using liquid chromatography and a single mix-mode solid-phase extraction cleanup for the simultaneous analysis of thiabendazole [2-(1,3-thiazol-4-yl)-1H-benzoimidazole], carbendazim [(methyl N-(1H-benzoimidazol-2-yl)-carbamate)] and 2-aminobenzimidaz... |
|
|
Novel small molecule bradykinin B1 receptor antagonists. Part 2: 5-membered diaminoheterocycles.
Bioorg. Med. Chem. Lett. 20(3) , 1229-32, (2010) Efforts to find new bradykinin B(1) receptor antagonists identified 2-aminobenzimidazole as a novel core. Subsequent transformation into five-membered diaminoheterocycle derivatives and their synthesis and SAR is described. This resulted in compounds with low... |
|
|
Removal of nonspecific binding proteins from cell and tissue extracts using 2-aminobenzimidazole-tethered affinity resin.
Pharmazie 66(9) , 662-5, (2011) Cellular drug target identification through affinity chromatography is often hindered by the quantity of nonspecific binders, such as cytoskeletal and heat shock proteins. Thus, we prepared a 2-aminobenzimidazole-tethered depletion resin designed for removal ... |
|
|
Zinc fingered: new compounds that thwart gram-positive biofilm formation by sequestering zinc.
ChemBioChem. 11(6) , 758-60, (2010)
|