3-Nitrophenol
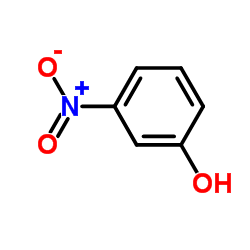
3-Nitrophenol structure
|
Common Name | 3-Nitrophenol | ||
|---|---|---|---|---|
| CAS Number | 554-84-7 | Molecular Weight | 139.109 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 277.6±0.0 °C at 760 mmHg | |
| Molecular Formula | C6H5NO3 | Melting Point | 89-95 °C | |
| MSDS | Chinese USA | Flash Point | 126.9±11.1 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
cIEF for rapid pKa determination of small molecules: a proof of concept.
Eur. J. Pharm. Sci. 63 , 14-21, (2014) A capillary isoelectric focusing (cIEF) method was developed for the determination of the ionization constants (pKa) of small molecules. Two approaches used to decrease the electroosmotic flow (EOF) were compared: (i) a hydroxypropylcellulose (HPC) coated cap... |
|
|
Effect of nitro substituent on electrochemical oxidation of phenols at boron-doped diamond anodes.
Chemosphere 78(9) , 1093-9, (2010) In order to investigate nitro-substitutent's effect on degradation of phenols at boron-doped diamond (BDD) anodes, cyclic voltammetries of three nitrophenol isomers: 2-nitrophenol (2NP), 3-nitrophenol (3NP) and 4-nitrophenol (4NP) were studied, and their bulk... |
|
|
Adsorption analysis of nitrophenol isomers on silver nanostructures by surface-enhanced spectroscopy.
J. Colloid. Interface Sci. 342(2) , 311-9, (2010) SEIRA, SERS, TPD and DFT were used to study 4-nitrophenol (4NP), 3-nitrophenol (3NP) and 2-nitrophenol (2NP) adsorption on nanoscale silver films/powder. SERS and DFT demonstrated that 4NP adsorbed as the 4-nitrophenolate ion. SEIRA results revealed that a 4N... |
|
|
Determination of partition coefficient and analysis of nitrophenols by three-phase liquid-phase microextraction coupled with capillary electrophoresis.
J. Sep. Sci. 33(14) , 2131-9, (2010) A three-phase hollow fiber liquid-phase microextraction method coupled with CE was developed and used for the determination of partition coefficients and analysis of selected nitrophenols in water samples. The selected nitrophenols were extracted from 14 mL o... |
|
|
Chromatographic peak resolution using Microsoft Excel Solver. The merit of time shifting input arrays.
J. Chromatogr. A. 1213(1) , 50-5, (2008) Resolution of overlapped chromatographic peaks is generally accomplished by modeling the peaks as Gaussian or modified Gaussian functions. It is possible, even preferable, to use actual single analyte input responses for this purpose and a nonlinear least squ... |
|
|
Haloalkane dehalogenases: steady-state kinetics and halide inhibition.
Biochemistry 38(18) , 5772-8, (1999) The substrate specificities and product inhibition patterns of haloalkane dehalogenases from Xanthobacter autotrophicus GJ10 (XaDHL) and Rhodococcus rhodochrous (RrDHL) have been compared using a pH-indicator dye assay. In contrast to XaDHL, RrDHL is efficien... |
|
|
Impact of nitrophenols on the photosynthetic electron transport chain and ATP content in Nostoc muscorum and Chlorella vulgaris.
Ecotoxicol. Environ. Saf. 58(2) , 256-9, (2004) Concentration-dependent inhibition of the photosynthetic electron transport chain (photosystem I (PS I), photosystem II (PS II) and whole chain reaction) and ATP content was observed in Nostoc muscorum and Chlorella vulgaris grown with o-nitrophenol, m-nitrop... |
|
|
Performance of throughout in-capillary derivatization capillary electrophoresis employing an on-line sample and run buffer loading device.
Electrophoresis 25(12) , 1810-6, (2004) We report on the effect on performance of varying the length of the capillary during throughout in-capillary derivatization (TICD) capillary electrophoresis (CE). Performance was evaluated by on-line coupling with a sample and CE runbuffer loading device that... |
|
|
Intermolecular interactions in solutions of some amino-nitro-benzene derivatives, studied by spectral means.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 73(2) , 257-62, (2009) The spectral shifts in the visible electronic absorption spectra of three amino-nitro-benzene derivatives in different solvents were correlated with the macroscopic parameters (refractive index and electric permittivity) of the solvents. The wavenumbers in th... |
|
|
Study on the aerobic biodegradability and degradation kinetics of 3-NP; 2,4-DNP and 2,6-DNP.
J. Hazard. Mater. 241-242 , 478-85, (2012) Four biodegradability tests (BOD(5)/COD ratio, production of carbon dioxide, relative oxygen uptake rate and relative enzymatic activity) were used to determine the aerobic biodegradability of 3-nitrophenol (3-NP), 2,4-dinitrophenol (2,4-DNP) and 2,6-dinitrop... |