3,4-Dihydroquinolin-2(1H)-one
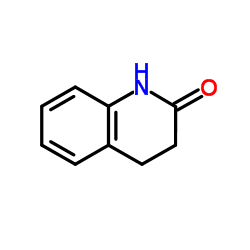
3,4-Dihydroquinolin-2(1H)-one structure
|
Common Name | 3,4-Dihydroquinolin-2(1H)-one | ||
|---|---|---|---|---|
| CAS Number | 553-03-7 | Molecular Weight | 147.174 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 328.1±31.0 °C at 760 mmHg | |
| Molecular Formula | C9H9NO | Melting Point | 165-167ºC | |
| MSDS | USA | Flash Point | 189.4±9.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Synthesis and biological evaluation of a novel sigma-1 receptor antagonist based on 3,4-dihydro-2(1H)-quinolinone scaffold as a potential analgesic.
Eur. J. Med. Chem. 79 , 216-30, (2014) The synthesis and sigma-1 receptor (1R) antagonist activity of a new series of 3,4-dihydro-2(1H)-quinolinone derivatives are reported. The new compounds were evaluated in vitro in sigma-1 and sigma-2 receptor-binding assays in guinea pig brain membranes. The ... |
|
|
Novel approach to 3,4-dihydro-2(1H)-quinolinone derivatives via cyclopropane ring expansion.
Org. Lett. 11(5) , 1043-5, (2009) N-(1'-Alkoxy)cyclopropyl-2-haloanilines are transformed to 3,4-dihydro-2((1)H)-quinolinones via palladium-catalyzed cyclopropane ring expansion. The reaction tolerates a variety of functional groups such as ester, nitrile, ether, and ketone groups. |
|
|
Synthesis of 4-amino-3, 4-dihydro-2 (1H)-quinolinones via β-lactam intermediates on the solid-phase. Pei Y, et al.
Tetrahedron Lett. 38(19) , 3349-52, (1997)
|
|
|
Selective removal of nitrogen from quinoline and petroleum by Pseudomonas ayucida IGTN9m. Kilbane JJ, et al.
Appl. Environ. Microbiol. 66(2) , 688-693, (2000)
|