1,3-dimethylbarbituric acid
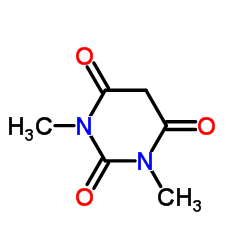
1,3-dimethylbarbituric acid structure
|
Common Name | 1,3-dimethylbarbituric acid | ||
|---|---|---|---|---|
| CAS Number | 769-42-6 | Molecular Weight | 156.139 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 228.1±23.0 °C at 760 mmHg | |
| Molecular Formula | C6H8N2O3 | Melting Point | 121-123 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 95.3±15.0 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
Organocatalytic Michael-Knoevenagel-hetero-Diels-Alder reactions: an efficient asymmetric one-pot strategy to isochromene pyrimidinedione derivatives.
Org. Lett. 14(2) , 448-51, (2012) Synthesis of isochromene pyrimidinedione derivatives having five stereocenters has been achieved by a one-pot Michael-Knoevenagel condensation-inverse-electron-demand hetero-Diels-Alder reaction of α, β-unsaturated aldehydes, olefinic nitroalkanes, and 1,3-di... |
|
|
Self-assembled Pd6 open cage with triimidazole walls and the use of its confined nanospace for catalytic Knoevenagel- and Diels-Alder reactions in aqueous medium.
Chemistry 18(39) , 12322-9, (2012) The two-component self-assembly of a 90° Pd(II) acceptor and a triimidazole donor led to the formation of a water-soluble semi-cylindrical cage with a hydrophobic cavity, which was separately crystallized with hydrophilic- and hydrophobic guests. The parent c... |
|
|
Multicomponent self-sorting of a Pd7 molecular boat and its use in catalytic Knoevenagel condensation.
Chem. Commun. (Camb.) 49(39) , 4307-9, (2013) Unique three-component self-assembly of a cis-blocked 90° Pd(II) acceptor with a mixture of tri- and tetra-imidazole donors led to the self-sorting of a Pd7 molecular boat with an internal nanocavity, which catalyses the Knoevenagel condensation of a series o... |
|
|
Synthesis of 5-aryl-1,3-dimethyl-6-(alkyl- or aryl-amino) furo [2,3-d]pyrimidine derivatives by reaction between isocyanides and pyridinecarbaldehydes in the presence of 1,3-dimethylbarbituric acid.
Mol. Divers. 15(1) , 227-31, (2011) 5-Aryl-6-(alkyl- or aryl-amino)-1,3-dimethylfuro [2,3-d]pyrimidine derivatives were obtained by in situ reaction alkyl or aryl isocyanides and pyridinecarbaldehyde derivatives in the presence of 1,3-dimethylbarbituric acid in dichloromethane without any prior... |
|
|
Sequential one-pot bimetallic Ir(III)/Pd(0) catalysed mono-/bis-alkylation and spirocyclisation processes of 1,3-dimethylbarbituric acid and allenes.
Chem. Commun. (Camb.) (48) , 5000-2, (2006) Microwave assisted indirect functionalization of alcohols with 1,3-dimethylbarbituric acid followed by spirocyclisation employing a sequential one-pot Ir(III)/Pd(0) catalysed process, involving the formation of three new C-C bonds, one spirocyclic ring and on... |
|
|
Experimental and computational thermochemical study of barbituric acids: structure-energy relationship in 1,3-dimethylbarbituric acid.
J. Phys. Chem. A 115(14) , 3167-73, (2011) This paper reports an experimental and computational thermochemical study on 1,3-dimethylbarbituric acid. The value of the standard (p° = 0.1 MPa) molar enthalpy of formation in the gas phase at T = 298.15 K has been determined. The energy of combustion was m... |
|
|
1, 3-Dimethylbarbituric Acid Argintaru OA.
e-EROS Encyclopedia of Reagents for Organic Synthesis. , (2011)
|