Diprophylline
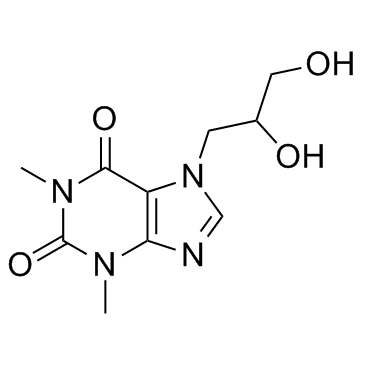
Diprophylline structure
|
Common Name | Diprophylline | ||
|---|---|---|---|---|
| CAS Number | 479-18-5 | Molecular Weight | 254.243 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 589.6±60.0 °C at 760 mmHg | |
| Molecular Formula | C10H14N4O4 | Melting Point | 161-162 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 310.4±32.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Prediction of volume of distribution values in human using immobilized artificial membrane partitioning coefficients, the fraction of compound ionized and plasma protein binding data
Eur. J. Med. Chem. 44 , 4455-60, (2009) We developed an improved Lombardeo's method (J. Med. Chem., 2004) for prediction of VD ss in human. With Elog D substituted by log k IAM, together with f i (7.4) (the fraction of compound ionized at pH 7.4) and log f u (logarithmic fraction of compound unboun... |
|
|
Development of a phospholipidosis database and predictive quantitative structure-activity relationship (QSAR) models.
Toxicol. Mech. Methods 18 , 217-27, (2008) ABSTRACT Drug-induced phospholipidosis (PL) is a condition characterized by the accumulation of phospholipids and drug in lysosomes, and is found in a variety of tissue types. PL is frequently manifested in preclinical studies and may delay or prevent the dev... |
|
|
Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds.
Drug Metab. Dispos. 36 , 1385-405, (2008) We present herein a compilation and trend analysis of human i.v. pharmacokinetic data on 670 drugs representing, to our knowledge, the largest publicly available set of human clinical pharmacokinetic data. This data set provides the drug metabolism scientist ... |
|
|
Measuring induction times and crystal nucleation rates.
Faraday Discuss. 179 , 199-214, (2015) A large variation is observed in induction times measured under equal conditions in 1 ml solutions. Ruling out experimental errors, this variation originates from the nucleation process. The induction time distribution is explained by the stochastic nature of... |
|
|
Physicochemical space for optimum oral bioavailability: contribution of human intestinal absorption and first-pass elimination.
J. Med. Chem. 53 , 1098-108, (2010) Oral bioavailability (F) is a product of fraction absorbed (Fa), fraction escaping gut-wall elimination (Fg), and fraction escaping hepatic elimination (Fh). In this study, using a database comprised of Fa, Fg, Fh, and F values for 309 drugs in humans, an ana... |
|
|
Prediction of dissolution time and coating thickness of sustained release formulations using Raman spectroscopy and terahertz pulsed imaging.
Eur. J. Pharm. Biopharm. 80 , 690-697, (2012) Raman spectroscopy was implemented successfully as a non-invasive and rapid process analytical technology (PAT) tool for in-line quantitative monitoring of functional coating. Coating experiments were performed at which diprophylline tablets were coated with ... |
|
|
Comparative study of the interactions between ovalbumin and three alkaloids by spectrofluorimetry.
Mol. Biol. Rep. 40(4) , 3409-18, (2013) The interaction between ovalbumin (OVA) and three purine alkaloids (caffeine, theophylline and diprophylline) was investigated by the aid of intrinsic and synchronous fluorescence, ultraviolet-vis absorbance, resonance light-scattering spectra and three-dimen... |
|
|
Separation of theophylline, caffeine and related drugs by normal-phase capillary electrochromatography.
Electrophoresis 20(12) , 2366-72, (1999) A capillary electrochromatography (CEC) method has been developed for the separation of theophylline, caffeine and five related drugs on a normal-phase column with UV or photodiode array detection. Several binary, ternary and quaternary mobile phase compositi... |
|
|
[Clinical observation on shufei granule in improving right ventricular function of patients with chronic pulmonary heart disease].
Zhongguo Zhong Xi Yi Jie He Za Zhi 26(8) , 732-5, (2006) To observe the effect of Shufei Granule (SG) on right ventricular function in patients with chronic pulmonary heart disease (CPHD).One hundred CPHD patients were randomly divided into two groups, the control group (n = 40) treated with fleroxacin 0.2 g twice ... |
|
|
Dissolution from solid lipid extrudates containing release modifiers
Int. J. Pharm. 412(1-2) , 77-84, (2011) The influence of different types of release modifiers on the dissolution from solid lipid extrudates was investigated. Diprophylline was extruded together with 45% tristearin and 5% (w/w) of a release modifier to suitable extrudates. Three groups of release m... |