3,3-Diphenylpropylamine
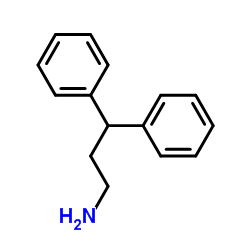
3,3-Diphenylpropylamine structure
|
Common Name | 3,3-Diphenylpropylamine | ||
|---|---|---|---|---|
| CAS Number | 5586-73-2 | Molecular Weight | 211.302 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 331.9±31.0 °C at 760 mmHg | |
| Molecular Formula | C15H17N | Melting Point | 29-31 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 155.2±17.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
(Bis)urea and (bis)thiourea inhibitors of lysine-specific demethylase 1 as epigenetic modulators.
J. Med. Chem. 53(14) , 5197-212, (2010) The recently discovered enzyme lysine-specific demethylase 1 (LSD1) plays an important role in the epigenetic control of gene expression, and aberrant gene silencing secondary to LSD1 overexpression is thought to contribute to the development of cancer. We re... |
|
|
Determination of the D- and L-enantiomers of modafinil in human plasma utilizing liquid-liquid extraction and high-performance liquid chromatography.
J. Chromatogr. B. Biomed. Sci. Appl. 730(1) , 1-7, (1999) Modafinil, DL-2-[(diphenylmethyl)sulfinyl]acetamide (Provigil), which is chiral at its sulfur atom, is a novel wake-promoting agent currently being developed as the racemate in the United States by Cephalon, Inc. In order to characterize the pharmacokinetic p... |
|
|
NPS 1506, a novel NMDA receptor antagonist and neuroprotectant. Review of preclinical and clinical studies.
Ann. N. Y. Acad. Sci. 890 , 450-7, (1999) NPS 1506 is a moderate affinity, uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist. NPS 1506 is neuroprotective in rodent models of ischemic stroke, hemorrhagic stroke, and head trauma, with a 2-hr window of opportunity. Neuroprotectant doses of N... |
|
|
In vitro inhibition of rat small intestinal absorption by lipophilic organic cations.
Biochim. Biophys. Acta 813(1) , 25-32, (1985) Cationic, lipid-soluble organic compounds may interfere with cation-mediated membrane transport processes. Thus, small intestinal absorption may be influenced by lipophilic organic cations. Therefore a series of arylalkylamines was studied in the concentratio... |
|
|
Binding of 2,2-diphenylpropylamine at the aldehyde site of bacterial luciferase increases the affinity of the reduced riboflavin 5'-phosphate site.
Biochemistry 20(19) , 5524-8, (1981) We have found a new class of inhibitors of the bacterial bioluminescence reaction, the N,N-diphenylalkylamines and acids. We have studied the action of one of these compounds 2,2-diphenylpropylamine. The amine was competitive with the long-chain aliphatic ald... |