6-Fluoro-2H-1,4-benzoxazin-3(4H)-one
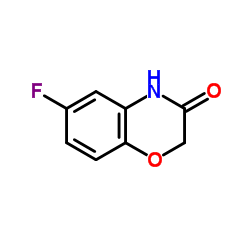
6-Fluoro-2H-1,4-benzoxazin-3(4H)-one structure
|
Common Name | 6-Fluoro-2H-1,4-benzoxazin-3(4H)-one | ||
|---|---|---|---|---|
| CAS Number | 398-63-0 | Molecular Weight | 167.137 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 322.9±42.0 °C at 760 mmHg | |
| Molecular Formula | C8H6FNO2 | Melting Point | 207-211ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 149.1±27.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
New herbicide models from benzoxazinones: aromatic ring functionalization effects.
J. Agric. Food Chem. 54(26) , 9843-51, (2006) The utility of benzoxazinones and some of their synthetic derivatives in the search for new leads for herbicide model development has been widely discussed. As the benzoxazinone skeleton contains three different potential areas for functionalization (C-2, N-4... |
|
|
Peroxidase-catalyzed halide ion oxidation.
Redox Rep. 5(4) , 169-71, (2000) The first complete mechanistic analysis of halide ion oxidation by a peroxidase was that of iodide oxidation by horseradish peroxidase. It was shown conclusively that a two-electron oxidation of iodide by compound I was occurring. This implied that oxygen ato... |
|
|
The proximate coupling constant, 5 J (H, CH3), and the torsional mobility of the thiomethyl group in some thioanisole derivatives. Schaefer T, et al.
Can. J. Chem. 69(4) , 620-624, (1991)
|
|
|
Catalytic hydrogenation of sulfur-containing nitrobenzene over Pd/C catalysts: In situ sulfidation of Pd/C for the preparation of Pd x S y catalysts. Zhang Q, et al.
Appl. Catal. A Gen. 497 , 17-21, (2015)
|
|
|
Photoelectrochemical reduction of meta-halonitrobenzenes and related species. Robert AW and George WJ.
J. Chem. Soc., Perkin Trans. II 8 , 1673-1677, (1995)
|