1-(3,5-Bis(trifluoromethyl)phenyl)ethanone
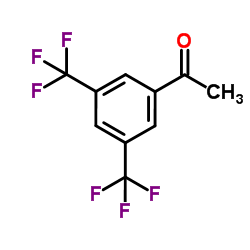
1-(3,5-Bis(trifluoromethyl)phenyl)ethanone structure
|
Common Name | 1-(3,5-Bis(trifluoromethyl)phenyl)ethanone | ||
|---|---|---|---|---|
| CAS Number | 30071-93-3 | Molecular Weight | 256.145 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 162.7±35.0 °C at 760 mmHg | |
| Molecular Formula | C10H6F6O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 82.2±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Inhibition of monoamine oxidase-B by 5H-indeno[1,2-c]pyridazines: biological activities, quantitative structure-activity relationships (QSARs) and 3D-QSARs.
J. Med. Chem. 38 , 3874-83, (1995) A large series (66 compounds) of indeno[1,2-c]pyridazin-5-ones (IPs) were synthesized and tested on their monoamine oxidase-A (MAO-A) and MAO-B inhibitory activity. All of the tested compounds acted preferentially on MAO-B displaying weak (nonmeasurable IC50 ... |
|
|
Asymmetric biocatalytic reduction of 3,5-bis(trifluoromethyl) acetophenone to (1R)-[3,5-bis(trifluoromethyl)phenyl] ethanol using whole cells of newly isolated Leifsonia xyli HS0904.
Appl. Microbiol. Biotechnol. 90(6) , 1897-904, (2011) A novel bacterial strain HS0904 was isolated from a soil sample using 3,5-bis(trifluoromethyl) acetophenone as the sole carbon source. This bacterial isolate can asymmetrically reduce 3,5-bis(trifluoromethyl) acetophenone to (1R)-[3,5-bis(trifluoromethyl)phen... |
|
|
Rhodium-catalyzed heterogeneous enantioselective hydrogenation of 3, 5-di-(trifluoromethyl)-acetophenone. Hess R, et al.
J. Mol. Catal. A: Chem. 212(1) , 205-9, (2004)
|