Prunit
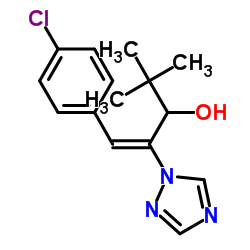
Prunit structure
|
Common Name | Prunit | ||
|---|---|---|---|---|
| CAS Number | 83657-22-1 | Molecular Weight | 291.776 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 474.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C15H18ClN3O | Melting Point | 153ºC | |
| MSDS | Chinese USA | Flash Point | 240.8±31.5 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Improved biological effects of uniconazole using porous hollow silica nanoparticles as carriers.
Pest Manag. Sci. 68(3) , 437-43, (2012) The aim of this work is to prepare a controlled-release formulation of uniconazole using porous hollow silica nanoparticles (PHSNs) as carrier, and to investigate the biological effects on rice growth.PHSNs with a shell thickness of ~15 nm and a particle size... |
|
|
Formation of embryogenic cell clumps from carrot epidermal cells is suppressed by 5-azacytidine, a DNA methylation inhibitor.
J. Plant Physiol. 162(1) , 47-54, (2005) Using a direct somatic embryogenesis system in carrot, we examined the role of DNA methylation in the change of cellular differentiation state, from somatic to embryogenic. 5-Azacytidine (aza-C), an inhibitor of DNA methylation suppressed the formation of emb... |
|
|
Enantiomeric resolution and growth-retardant activity in rice seedlings of uniconazole.
J. Agric. Food Chem. 60(1) , 160-4, (2012) The increasing application of chiral pesticides has enhanced interest in their enantioselectivity. However, little relevant information is currently available for enantioselective activity of chiral plant growth regulators. In an attempt to screen active enan... |
|
|
Involvement of gibberellin in tracheary element differentiation and lignification in Zinnia elegans xylogenic culture.
Protoplasma 228(4) , 179-87, (2006) Gibberellin (GA) is considered an important growth regulator involved in many aspects of plant development. However, little is known about the relationship between GA and lignification. In this study, we analyzed the role of GA in tracheary element (TE) diffe... |
|
|
Plants with increased expression of ent-kaurene oxidase are resistant to chemical inhibitors of this gibberellin biosynthesis enzyme.
Plant Cell Physiol. 46(2) , 284-91, (2005) The gibberellin (GA) biosynthetic pathway includes the three-step oxidation of ent-kaurene to ent-kaurenoic acid, catalyzed by the enzyme ent-kaurene oxidase (KO). Arabidopsis plants overexpressing the KO cDNA under the control of the cauliflower mosaic virus... |
|
|
Metabolism of uniconazole-P in water-sediment systems under illumination.
Environ. Toxicol. Chem. 25(2) , 310-6, (2006) Aerobic soil metabolism of uniconazole-P ([S]-E-1-[4-chlorophenyl]-4,4-dimethyl-2-[1,2,4-triazole-1-yl]-penten-3-ol) and the effect of illumination on metabolic profiles were studied in the water-sediment system when spiked to water. Uniconazole-P was gradual... |
|
|
The leaf morphologies of the subtropical rheophyte Solenogyne mikadoi and its temperate relative S. bellioides (Asteraceae) are affected differently by plant hormones and their biosynthesis inhibitors.
J. Plant Res. 118(3) , 181-6, (2005) Solenogyne mikadoi is a subtropical rheophyte endemic to the Ryukyu Archipelago that develops rosette leaves 2-3 cm in diameter. In contrast, the other three species of this genus all occur in temperate grasslands of Australia and develop rosette leaves about... |
|
|
Quantitative analysis of three chiral pesticide enantiomers by high-performance column liquid chromatography.
J. AOAC Int. 91(5) , 1007-12, (2008) Methods for the enantiomeric quantitative determination of 3 chiral pesticides, paclobutrazol, myclobutanil, and uniconazole, and their residues in soil and water are reported. An effective chiral high-performance liquid chromatographic (HPLC)-UV method using... |
|
|
Uniconazole residue and decline in wheat and soil under field application.
Bull. Environ. Contam. Toxicol. 90(4) , 499-503, (2013) Uniconazole residue dynamics and final residues in supervised field trials at GAP conditions were studied. The residue levels and dissipation rate of uniconazole was detected by LC-MS. At fortification levels of 0.04, 0.2 and 2 mg kg(-1), recoveries ranged fr... |
|
|
The chiral separation of triazole pesticides enantiomers by amylose-tris (3,5-dimethylphenylcarbamate) chiral stationary phase.
J. Chromatogr. Sci. 46(9) , 787-92, (2008) The amylose-tris(3,5-dimethylphenylcarbamate) chiral stationary phase was synthesized and used to separate the enantiomers of triazole pesticides by high-performance liquid chromatography. The mobile phase was n-hexane-isopropanol applying a flow rate of 1.0 ... |