Metoprolol Tartrate
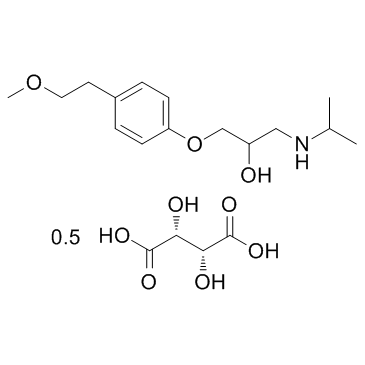
Metoprolol Tartrate structure
|
Common Name | Metoprolol Tartrate | ||
|---|---|---|---|---|
| CAS Number | 56392-17-7 | Molecular Weight | 342.41 | |
| Density | N/A | Boiling Point | 398.6ºC at 760 mmHg | |
| Molecular Formula | C17H28NO6 | Melting Point | 120ºC | |
| MSDS | Chinese USA | Flash Point | 194.9ºC | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
|
Environmental friendly method for urban wastewater monitoring of micropollutants defined in the Directive 2013/39/EU and Decision 2015/495/EU.
J. Chromatogr. A. 1418 , 140-9, (2015) The fate and removal of organic micropollutants in the environment is a demanding issue evidenced by the recent European policy. This work presents an analytical method for the trace quantification of 37 micropollutants including Priority Substances (Directiv... |
|
|
Human and simulated intestinal fluids as solvent systems to explore food effects on intestinal solubility and permeability.
Eur. J. Pharm. Sci. 63 , 178-86, (2014) The mixed micelles and vesicles present in the intraluminal environment of the postprandial state exhibit suitable solubilizing capacities for lipophilic drugs. This increase in solubility, however, is accompanied by a decrease in the free fraction caused by ... |
|
|
Evaluation of the effect of TM208 on the activity of five cytochrome P450 enzymes using on-line solid-phase extraction HPLC-DAD: a cocktail approach.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 923-924 , 29-36, (2013) A rapid, simple, and sensitive on-line solid-phase extraction HPLC-DAD method for simultaneous evaluation of the activity of five CYP450 isoforms (CYP1A2, CYP2C19, CYP2D6, CYP2E1 and CYP3A4) in vivo has been developed and validated. The five specific probe su... |
|
|
Enantiomeric fraction evaluation of pharmaceuticals in environmental matrices by liquid chromatography-tandem mass spectrometry.
J. Chromatogr. A. 1363 , 226-235, (2014) The interest for environmental fate assessment of chiral pharmaceuticals is increasing and enantioselective analytical methods are mandatory. This study presents an enantioselective analytical method for the quantification of seven pairs of enantiomers of pha... |
|
|
Low contrast and radiation dose coronary CT angiography using a 320-row system and a refined contrast injection and timing method.
J. Cardiovasc. Comput. Tomogr. 9(1) , 19-27, (2015) Among CT scanners, 320-row instruments feature decreased photon energy and yield strong contrast enhancement. Consequently, the contrast medium (CM) dose can be reduced. The results of low-tube-voltage coronary CT angiography (CCTA) performed on 320-row scann... |
|
|
Optimization of sustained release matrix tablet of metoprolol succinate using central composite design.
Pak. J. Pharm. Sci. 26(5) , 929-37, (2013) The present study was performed to optimize the formulation of metoprolol succinate (MS) sustained release tablets using hydroxypropyl methylcellulose (HPMC) and sodium alginate (SA) as the matrix combination. After investigating the effects of various parame... |
|
|
In vitro to in vivo profiling: an easy idea for biowaiver study.
Acta Pol. Pharm. 70(5) , 873-5, (2013) The aim of this article was to assess and apply the in vitro to in vivo profiling (IVIVP), a new biowaiver approach, in designing a product with specific release pattern. The IVIVP was established by plotting the observed and predicted plasma drug concentrati... |
|
|
Ex vivo correlation of the permeability of metoprolol across human and porcine buccal mucosa.
J. Pharm. Sci. 103(7) , 2053-61, (2014) The pH partition theory proposes a correlation between fraction of unionized drug substance and permeability. The aim of this study was to compare the permeability of metoprolol and mannitol in ex vivo human and porcine buccal mucosa models at varying pH to v... |
|
|
The complexity of intestinal permeability: Assigning the correct BCS classification through careful data interpretation.
Eur. J. Pharm. Sci. 61 , 11-7, (2014) While the solubility parameter is fairly straightforward when assigning BCS classification, the intestinal permeability (Peff) is more complex than generally recognized. In this paper we emphasize this complexity through the analysis of codeine, a commonly us... |
|
|
Evaluation of a three compartment in vitro gastrointestinal simulator dissolution apparatus to predict in vivo dissolution.
J. Pharm. Sci. 103(11) , 3416-22, (2014) In vitro dissolution tests are performed for new formulations to evaluate in vivo performance, which is affected by the change of gastrointestinal (GI) physiology, in the GI tract. Thus, those environmental changes should be introduced to an in vitro dissolut... |