2,4-Hexadienal
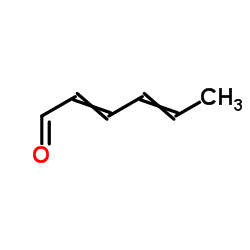
2,4-Hexadienal structure
|
Common Name | 2,4-Hexadienal | ||
|---|---|---|---|---|
| CAS Number | 142-83-6 | Molecular Weight | 96.127 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 155.9±9.0 °C at 760 mmHg | |
| Molecular Formula | C6H8O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 67.8±0.0 °C | |
| Symbol |


GHS05, GHS06 |
Signal Word | Danger | |
|
Volatile Compounds from Grape Skin, Juice and Wine from Five Interspecific Hybrid Grape Cultivars Grown in Québec (Canada) for Wine Production.
Molecules 20 , 10980-1016, (2015) Developed from crosses between Vitis vinifera and North American Vitis species, interspecific hybrid grape varieties are becoming economically significant in northern areas, where they are now extensively grown for wine production. However, the varietal diffe... |
|
|
Comparison of Aroma-Active Volatiles in Oolong Tea Infusions Using GC-Olfactometry, GC-FPD, and GC-MS.
J. Agric. Food Chem. 63 , 7499-510, (2015) The aroma profile of oolong tea infusions (Dongdingwulong, DDWL; Tieguanyin, TGY; Dahongpao, DHP) were investigated in this study. Gas chromatography-olfactometry (GC-O) with the method of aroma intensity (AI) was employed to investigate the aroma-active comp... |
|
|
Characterization of Taenia solium cysticerci microsomal glutathione S-transferase activity.
Parasitol. Res. 101(5) , 1373-81, (2007) Glutathione S-transferase activity has been shown to be associated with the microsomal fraction of Taenia solium. Electron microscopy and subcellular enzyme markers indicate the purity of the microsomal fraction that contains the glutathione S-transferase act... |
|
|
NTP toxicology and carcinogensis Studies of 2,4-hexadienal (89% trans,trans isomer, CAS No. 142-83-6; 11% cis,trans isomer) (Gavage Studies).
Natl. Toxicol. Program Tech. Rep. Ser. (509) , 1-290, (2003) 2,4-Hexadienal, a colorless to yellow liquid with a pungent "green" or citrus odor, is used as a food additive for flavor enhancement, as a fragrance agent, as a starting material or intermediate in synthetic reactions in the chemical and pharmaceutical indus... |
|
|
Cell spreading on collagen that has been exposed to reactive aldehydes.
Biochem. Soc. Trans. 20(4) , 369S, (1992)
|
|
|
exo-Selective asymmetric Diels-Alder reaction of 2,4-dienals and nitroalkenes by trienamine catalysis.
Angew. Chem. Int. Ed. Engl. 50(37) , 8638-41, (2011)
|
|
|
The atmospheric photolysis of E-2-hexenal, Z-3-hexenal and E,E-2,4-hexadienal.
Phys. Chem. Chem. Phys. 8(44) , 5236-46, (2006) The atmospheric photolysis of E-2-hexenal, Z-3-hexenal and E,E-2,4-hexadienal has been investigated at the large outdoor European Photoreactor (EUPHORE) in Valencia, Spain. E-2-Hexenal and E,E-2,4-hexadienal were found to undergo rapid isomerization to produc... |
|
|
Alpha,beta-unsaturated carbonyl compounds: induction of oxidative DNA damage in mammalian cells.
Mutagenesis 18(5) , 465-70, (2003) Alpha,beta-unsaturated carbonyl compounds occur in food and other environmental media. Due to their reactivity with cellular nucleophiles (e.g. Michael adduct formation with DNA bases and with glutathione) they might represent a potential health risk. In this... |
|
|
Glutathione S-transferase pi expression in forestomach carcinogenesis process induced by gavage-administered 2,4-hexadienal in the F344 rat.
Arch. Toxicol. 75(10) , 618-24, (2001) 2,4-Hexadienal (2,4-Hx), an unsaturated aldehyde formed by in vivo and in vitro peroxidation of unsaturated lipid induced, in National Toxicology Program (NTP) gavage studies of F344 rats, forestomach hyperplasia in 13-week and 2-year exposures and squamous p... |
|
|
Forestomach tumor induction by 2,4-hexadienal in F344N rats and B6C3F1 mice.
Arch. Toxicol. 77(9) , 511-20, (2003) 2,4-Hexadienal (2,4-Hx) was studied for its toxicity and carcinogenicity because of its alpha, beta-unsaturated aldehyde structure and potential link between exposure to lipid peroxidation products in the diet and human malignancies. Male and female F344N rat... |