Methyl 3,3,3-trifluoro-2-oxopropanoate
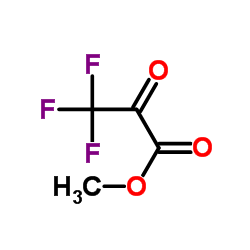
Methyl 3,3,3-trifluoro-2-oxopropanoate structure
|
Common Name | Methyl 3,3,3-trifluoro-2-oxopropanoate | ||
|---|---|---|---|---|
| CAS Number | 13089-11-7 | Molecular Weight | 156.060 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 58.5±40.0 °C at 760 mmHg | |
| Molecular Formula | C4H3F3O3 | Melting Point | 84-86ºC | |
| MSDS | Chinese USA | Flash Point | -9.0±22.2 °C | |
| Symbol |

GHS02 |
Signal Word | Warning | |
|
Methyl 3,3,3-trifluoropyruvate: an improved procedure starting from hexafluoropropene-1, 2-oxide; identification of byproducts. Dolenský B, et al.
J. Fluor. Chem. 115(1) , 67-74, (2002)
|
|
|
Methyl 3, 3, 3-trifluoropyruvate hemiaminals: Stability and transaminations. Dolenský B, et al.
J. Fluor. Chem. 126(5) , 745-751, (2005)
|
|
|
Fluorinated butanolides and butenolides: Part 8. 2-(Trifluoromethyl) butan-4-olides by synthesis from methyl 3, 3, 3-trifluoropyruvate as building block. Paleta O, et al.
J. Fluor. Chem. 111(2) , 175-184, (2001)
|
|
|
Fluorinated butanolides and butenolides: Part 9. Synthesis of 2-(trifluoromethyl) butan-4-olides by Wittig reaction using methyl 3, 3, 3-trifluoropyruvate. Palecek J, et al.
J. Fluor. Chem. 113(2) , 177-183, (2002)
|
|
|
A convenient synthesis of 4-trifluoromethyl-(2H)-pyridazin-3-ones from methyl 3, 3, 3-trifluoropyruvate. Sibgatulin DA, et al.
Synlett 12 , 1907-1911, (2005)
|
|
|
Fluorine-Sacrificial Cyclizations as an Access to 5-Fluoropyrazoles. Volle JN and Schlosser M.
European J. Org. Chem. 2000(5) , 823-828, (2000)
|
Journals:
More...