3,5-di-t-Butyl-4-hydroxyanisole
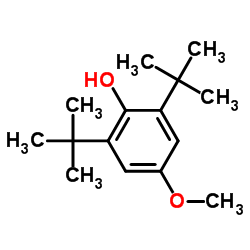
3,5-di-t-Butyl-4-hydroxyanisole structure
|
Common Name | 3,5-di-t-Butyl-4-hydroxyanisole | ||
|---|---|---|---|---|
| CAS Number | 489-01-0 | Molecular Weight | 236.350 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 297.5±40.0 °C at 760 mmHg | |
| Molecular Formula | C15H24O2 | Melting Point | 102-106 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 78.8±12.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Structural characterization of lignin: a potential source of antioxidants guaiacol and 4-vinylguaiacol.
Int. J. Biol. Macromol. 75 , 58-66, (2015) The structure of lignin obtained from the ozone and soaking aqueous ammonia pretreatment of wheat straw has been characterized utilizing chemical analytical methods in order to reveal its antioxidant characteristics, including attenuated total reflectance-Fou... |
|
|
Cellular apoptosis and cytotoxicity of phenolic compounds: a quantitative structure-activity relationship study.
J. Med. Chem. 48 , 7234-42, (2005) In this comprehensive study on the caspase-mediated apoptosis-inducing effect of 51 substituted phenols in a murine leukemia cell line (L1210), we determined the concentrations needed to induce caspase activity by 50% (I50) and utilized these data to develop ... |
|
|
Discovery of novel SERCA inhibitors by virtual screening of a large compound library.
Eur. J. Med. Chem. 46 , 1512-23, (2011) Two screening protocols based on recursive partitioning and computational ligand docking methodologies, respectively, were employed for virtual screens of a compound library with 345,000 entries for novel inhibitors of the enzyme sarco/endoplasmic reticulum c... |
|
|
Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols.
Bioorg. Med. Chem. 17 , 3207-11, (2009) The inhibition of two human cytosolic carbonic anhydrase (hCA, EC 4.2.1.1) isozymes I and II, with a series of phenol derivatives was investigated by using the esterase assay, with 4-nitrophenyl acetate as substrate. 2,6-Dimethylphenol, 2,6-diisopropylphenol ... |
|
|
In(OTf)3-catalyzed tandem nucleophilic addition and cyclization of ortho-alkynylarylaldimines to 1,2-dihydroisoquinolines.
Angew. Chem. Int. Ed. Engl. 45(23) , 3822-5, (2006)
|
|
|
Polypyrroles as antioxidants: kinetic studies on reactions of bilirubin and biliverdin dimethyl esters and synthetic model compounds with peroxyl radicals in solution. Chemical calculations on selected typical structures.
J. Org. Chem. 71(1) , 22-30, (2006) [reaction: see text] Rate constants for hydrogen-atom transfer (HAT) from bilirubin dimethyl ester (BRDE) and biliverdin dimethyl ester (BVDE) to peroxyl radicals during inhibited autoxidation of styrene initiated by azo-bisisobutyronitrile (AIBN) were k(inh)... |
|
|
Antioxidant activity of depsides and depsidones.
Phytochemistry 37(6) , 1585-7, (1994) The antioxidant activity of lichenic metabolites, depsides and depsidones, was assessed by their effects as inhibitors of rat brain homogenate auto-oxidation and beta-carotene oxidation. The results obtained in both systems indicate that lichenic metabolites ... |