4-METHOXYPHENYLACETYL CHLORIDE
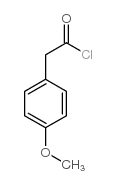
4-METHOXYPHENYLACETYL CHLORIDE structure
|
Common Name | 4-METHOXYPHENYLACETYL CHLORIDE | ||
|---|---|---|---|---|
| CAS Number | 4693-91-8 | Molecular Weight | 184.62000 | |
| Density | 1.208 g/mL at 25 °C(lit.) | Boiling Point | 143 °C10 mm Hg(lit.) | |
| Molecular Formula | C9H9ClO2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | >230 °F | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Synthesis of substituted spiro [4.5] deca-3, 6, 9-triene-2, 8-diones: an expeditious route to the spiro [4.5] decane terpene skeleton. Haack RA and Beck KR.
Tetrahedron Lett. 30(13) , 16505-8, (1989)
|
|
|
Synthesis of higenamine, A cardiotonic principle of aconite root. Chang K-C, et al.
Arch. Pharm. Res. 7(2) , 133-36, (1984)
|
|
|
Synthesis and dopamine receptor selectivity of the benzyltetrahydroisoquinoline, (R)-(+)-nor-roefractine.
J. Nat. Prod. 61(6) , 709-12, (1998) (R)-(+)-nor-Roefractine (1) was synthesized by the Bischler-Napieralski route, using asymmetric reduction of the 1, 2-didehydro precursor imine with sodium (S)-N-CBZ-prolinyloxyborohydride. Compound 1 was able to displace [3H]-raclopride (a D2 dopamine recept... |
|
|
Novel naphthyridines are histamine H3 antagonists and serotonin reuptake transporter inhibitors.
Bioorg. Med. Chem. Lett. 17(9) , 2566-9, (2007) A series of novel tetrahydronaphthyridine-based histamine H(3) ligands that have serotonin reuptake transporter inhibitor activity is described. The 1,2,3,4-tetrahydro-2,6-naphthyridine scaffold is assembled via the addition of a nitrostyrene to a metalated p... |