1-Piperidinamine
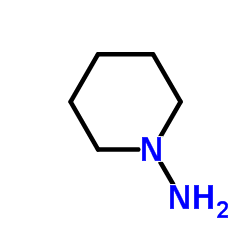
1-Piperidinamine structure
|
Common Name | 1-Piperidinamine | ||
|---|---|---|---|---|
| CAS Number | 2213-43-6 | Molecular Weight | 100.162 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 146.9±0.0 °C at 760 mmHg | |
| Molecular Formula | C5H12N2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 36.1±0.0 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Warning | |
|
Topoisomerase II from Human Malaria Parasites: EXPRESSION, PURIFICATION, AND SELECTIVE INHIBITION.
J. Biol. Chem. 290 , 20313-24, (2015) Historically, type II topoisomerases have yielded clinically useful drugs for the treatment of bacterial infections and cancer, but the corresponding enzymes from malaria parasites remain understudied. This is due to the general challenges of producing malari... |
|
|
Inhibition of metabolism--mediated cytotoxicity by 1,1-disubstituted hydrazines in mouse mastocytoma cells (line P815).
Adv. Exp. Med. Biol. 136 Pt B , 1067-75, (1981)
|
|
|
Aerial oxidation of hydrazines to nitrosamines.
Environ. Mol. Mutagen. 17(1) , 59-62, (1991) When 1,1-dimethylhydrazine and N-aminopiperidine were deliberately exposed to air substantial amounts of the corresponding carcinogenic nitrosamines were formed. Unoxidized samples of 1,1-dimethylhydrazine were not mutagenic while oxidized samples (which cont... |
|
|
1,3-disubstituted 4-aminopiperidines as useful tools in the optimization of the 2-aminobenzo[a]quinolizine dipeptidyl peptidase IV inhibitors.
Bioorg. Med. Chem. Lett. 17(11) , 2966-70, (2007) In a search for novel DPP-IV inhibitors, 2-aminobenzo[a]quinolizines were identified as submicromolar HTS hits. Due to the difficult synthetic access to this compound class, 1,3-disubstituted 4-aminopiperidines were used as model compounds for optimization. T... |