Tris(dibenzylideneacetone)dipalladium(0)
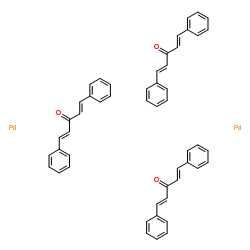
Tris(dibenzylideneacetone)dipalladium(0) structure
|
Common Name | Tris(dibenzylideneacetone)dipalladium(0) | ||
|---|---|---|---|---|
| CAS Number | 51364-51-3 | Molecular Weight | 915.72 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C51H42O3Pd2 | Melting Point | 152-155°C | |
| MSDS | USA | Flash Point | N/A | |
| Symbol |

GHS08 |
Signal Word | Warning | |
|
Efficient syntheses of [¹¹C]zidovudine and its analogs by convenient one-pot palladium(0)-copper(I) co-mediated rapid C-[¹¹C]methylation.
J. Labelled Comp. Radiopharm. 57(8) , 540-9, (2014) The nucleosides zidovudine (AZT), stavudine (d4T), and telbivudine (LdT) are approved for use in the treatment of human immunodeficiency virus (HIV) and hepatitis B virus (HBV) infections. To promote positron emission tomography (PET) imaging studies on their... |
|
|
Carborane-derived local anesthetics are isomer dependent.
ChemMedChem 10(1) , 62-7, (2014) Clinically there is a need for local anesthetics with a greater specificity of action on target cells and longer duration. We have synthesized a series of local anesthetic derivatives we call boronicaines in which the aromatic phenyl ring of lidocaine was rep... |
|
|
Palladium-catalyzed conversion of aryl and vinyl triflates to bromides and chlorides.
J. Am. Chem. Soc. 132 , 14076, (2010) The palladium-catalyzed conversion of aryl and vinyl triflates to aryl and vinyl halides (bromides and chlorides) has been developed using dialkylbiaryl phosphine ligands. A variety of aryl, heteroaryl, and vinyl halides can be prepared via this method in goo... |
|
|
Palladium-catalyzed β arylation of carboxylic esters.
Angew. Chem. Int. Ed. Engl. 49 , 7261, (2010)
|
|
|
Asymmetric synthesis of (-)-aurantioclavine via palladium-catalyzed intramolecular allylic amination.
Org. Lett. 16(3) , 996-9, (2014) The total synthesis of (-)-aurantioclavine (1) was accomplished based on an intramolecular asymmetric amination of allyl carbonate 3 containing a p-tosylamide group. The reaction using tris(dibenzylideneacetone)dipalladium(0), tBu-phosphinooxazoline, and Bu4N... |
|
|
The elusive structure of Pd2(dba)3. Examination by isotopic labeling, NMR spectroscopy, and X-ray diffraction analysis: synthesis and characterization of Pd2(dba-Z)3 complexes.
J. Am. Chem. Soc. 135(22) , 8388-99, (2013) Pd(0)2(dba)3 (dba = E,E-dibenzylidene acetone) is the most widely used Pd(0) source in Pd-mediated transformations. Pd(0)2(dba-Z)3 (Z = dba aryl substituents) complexes exhibit remarkable and differential catalytic performance in an eclectic array of cross-co... |
|
|
Palladium-catalyzed asymmetric synthesis of allylic fluorides.
J. Am. Chem. Soc. 132 , 17402, (2010) The enantioselective fluorination of readily available cyclic allylic chlorides with AgF has been accomplished using a Pd(0) catalyst and Trost bisphosphine ligand. The reactions proceed with unprecedented ease of operation for Pd-mediated nucleophilic fluori... |
|
|
Pd(0)-Catalyzed carbonylation of 1,1-dichloro-1-alkenes, a new selective access to Z-α-chloroacrylates.
Chem. Commun. (Camb.) 46 , 7810, (2010) A novel and fully chemo- and stereoselective three component strategy leading to Z-α-chloroacrylates by a Pd(0)-catalyzed reaction of CO (1 atm) with 1,1-dichloro-1-alkenes and various alcohols is disclosed. This catalytic approach compares favourably with th... |
|
|
Littke, A. F.; Dai, C.; Fu, G.C.
J. Am. Chem. Soc. 122 , 4020, (2000)
|
|
|
Kawatsura, M.; Hartwig, J. F.
J. Am. Chem. Soc. 121 , 1473, (1999)
|