Cyanoacetic acid
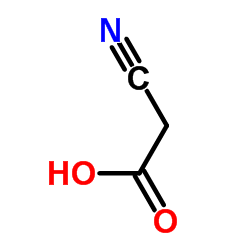
Cyanoacetic acid structure
|
Common Name | Cyanoacetic acid | ||
|---|---|---|---|---|
| CAS Number | 372-09-8 | Molecular Weight | 85.061 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 318.5±0.0 °C at 760 mmHg | |
| Molecular Formula | C3H3NO2 | Melting Point | 65 °C | |
| MSDS | Chinese USA | Flash Point | 107.8±0.0 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
Identification of two urinary metabolites of rats treated with acrylonitrile; influence of several inhibitors on the mutagenicity of those urines.
Toxicol. Lett. 7(4-5) , 321-7, (1981) Urines collected from rats injected with acrylonitrile (ACN) were mutagenic towards Salmonella typhimurium TA1530; this activity was reduced when the animals were pretreated by pyrazole (inhibitor of alcohol dehydrogenase) and suppressed after pretreatment ei... |
|
|
A copper(I)-catalyzed reaction of 2-(2-ethynylphenyl)oxirane, sulfonyl azide, with 2-isocyanoacetate.
Chem. Commun. (Camb.) 48(71) , 8973-5, (2012) A novel and straightforward synthetic protocol for the efficient construction of 3',5'-dihydro-1H-spiro[benzo[d]oxepine-2,4'-imidazoles] through a copper(I)-catalyzed reaction between 2-(2-ethynylphenyl)oxirane, sulfonyl azide, and 2-isocyanoacetate is descri... |
|
|
Metabolism of 3,3'-iminodipropionitrile and deuterium-substituted analogs: potential mechanisms of detoxification and activation.
Toxicol. Appl. Pharmacol. 124(1) , 59-66, (1994) 3,3'-Iminodipropionitrile (IDPN), a neurotoxicant that causes an excitatory CNS syndrome and a proximal axonopathy, is metabolized to beta-aminopropionitrile (BAPN), cyanoacetic acid (CAA), and beta-alanine (beta-ala) in rats. None of these metabolites are ne... |
|
|
Synthesis and modification of dibenzylglycine derivatives via the Suzuki-Miyaura cross-coupling reaction.
J. Pept. Res. 64(2) , 72-85, (2004) The objective of this paper is to describe in details of various available methods to prepare C(alpha,alpha)-dibenzylglycine (Dbzg) and then include our work involving the synthesis of side chain Dbzg derivatives. alpha,alpha-Disubstituted amino acids (alpha,... |
|
|
The simultaneous determination of chloride, nitrate and sulphate by isotachophoresis using bromide as a leading ion
Talanta 75(3) , 841-5, (2008) A new method has been devised to allow the determination of small inorganic anions using isotachophoresis. This method makes use of indium(III) as a counter ion to manipulate the effective mobilities of inorganic anion species by means of complexation reactio... |
|
|
The urotoxic effects of N,N'-dimethylaminopropionitrile. 2. In vivo and in vitro metabolism.
Toxicol. Appl. Pharmacol. 110(1) , 61-9, (1991) The urotoxicity and metabolism of N,N'-dimethylaminopropionitrile (DMAPN) were investigated in male Sprague-Dawley rats. Animals treated with 525 mg DMAPN/kg or equimolar doses of commercially available potential DMAPN metabolites showed varying levels of uri... |
|
|
Design of a versatile multicomponent reaction leading to 2-amino-5-ketoaryl pyrroles.
Chem. Biol. Drug Des. 75(3) , 277-83, (2010) The design of an unprecedented multicomponent reaction to and synthesis of 2-amino-5-ketoaryl pyrroles are described. The compounds (14 examples) can be synthesized by reacting aminoacetophenone sulfonamides, (hetero)aromatic aldehydes, and malonodinitrile or... |
|
|
Synthesis of bis-armed amino acid derivatives via the alkylation of ethyl isocyanoacetate and the Suzuki-Miyaura cross-coupling reaction.
Amino Acids 32(3) , 387-94, (2007) Two synthetic routes to bis-armed-alpha-amino acid derivatives are described. The first route involves alkylation of dibromo derivatives with ethyl isocyanoacetate under phase-transfer catalysis (PTC) conditions. The second route uses a palladium-mediated Suz... |
|
|
Acylation of heteroaromatic amines: facile and efficient synthesis of a new class of 1,2,3-triazolo[4,5-b]pyridine and pyrazolo[4,3-b]pyridine derivatives.
Molecules 16(5) , 3723-39, (2011) 1,2,3-Triazolo[4,5-b]pyridines and pyrazolo[4,3-b]pyridines can be readily prepared via cyanoacetylation reactions of 5-amino-1,2,3-triazoles 1a,b and 4-amino- pyrazole 2 followed by subsequent cyclization of the formed cyanoacetamides. Reactions of amines 1a... |
|
|
Studies on 3-oxoalkanenitriles: novel rearrangement reactions observed in studies of the chemistry of 3-heteroaroyl-3-oxoalkanenitriles as novel routes to 2-dialkylaminopyridines.
Molecules 17(1) , 897-909, (2012) 3-Aroyl and 3-heteroaroyl substituted 3-oxoalkanenitriles were synthesized by the reactions of activated aromatic and hetero-aromatic substances with cyanoacetic acid in the presence of acetic anhydride. As part of studies focusing on the preparation of cyano... |