3-Methoxy-2-cyclopenten-1-one
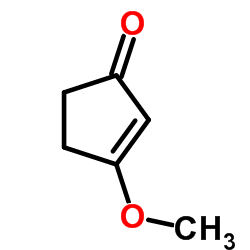
3-Methoxy-2-cyclopenten-1-one structure
|
Common Name | 3-Methoxy-2-cyclopenten-1-one | ||
|---|---|---|---|---|
| CAS Number | 4683-50-5 | Molecular Weight | 112.127 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 214.9±29.0 °C at 760 mmHg | |
| Molecular Formula | C6H8O2 | Melting Point | 49-53ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 96.5±17.8 °C | |
|
Synthesis and structure-activity relationship of cyclopentenone oximes as novel inhibitors of the production of tumor necrosis factor-α.
Bioorg. Med. Chem. Lett. 24(13) , 2807-10, (2014) 3-Alkyl-2-aryl-2-cyclopenten-1-one oxime derivatives (1) were studied as a novel class of inhibitors of tumor necrosis factor α (TNF-α) with regard to synthesis and in vitro SAR inhibition of TNF-α. The in vitro IC50 values of these compounds in rat and human... |
|
|
Stereoselective α-quaternization of 3-methoxycycloalk-2-enones via 1,4-diastereoinduction of alkoxy dienolates.
J. Org. Chem. 77(2) , 1202-7, (2012) The alkylation of dienolates generated from 3-methoxycycloalk-2-enones having a 3'-hydroxyl alkenyl chain provides the corresponding quaternized cycloalkenones in a highly diastereoselective manner. The high degree of stereocontrol in the α-quaternization pos... |
|
|
Expanding the scope of asymmetric electrophilic atom-transfer reactions: titanium- and ruthenium-catalyzed hydroxylation of beta-ketoesters.
Proc. Natl. Acad. Sci. U. S. A. 101(16) , 5810-4, (2004) The enantioselective formation of a quaternary stereogenic center coinciding with a hydroxylation process is a very rare reaction from a homogeneous catalysis point of view. Indeed, to our knowledge, no asymmetric transition-metal-catalyzed direct hydroxylati... |
|
|
Discovery of orally efficacious melanin-concentrating hormone receptor-1 antagonists as antiobesity agents. Synthesis, SAR, and biological evaluation of bicyclo [3.1. 0] hexyl ureas. McBriar MD, et al.
J. Med. Chem. 49(7) , 1202-1207, (2012)
|