beryllium oxide
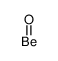
beryllium oxide structure
|
Common Name | beryllium oxide | ||
|---|---|---|---|---|
| CAS Number | 1304-56-9 | Molecular Weight | 25.01160 | |
| Density | 3.0100g/cm3 | Boiling Point | 4300ºC | |
| Molecular Formula | BeO | Melting Point | 2575ºC | |
| MSDS | Chinese USA | Flash Point | 4300°C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
|
Extraction of beryllium from refractory beryllium oxide with dilute ammonium bifluoride and determination by fluorescence: a multiparameter performance evaluation.
J. Occup. Environ. Hyg. 6(12) , 735-44, (2009) Beryllium exposure can cause a number of deleterious health effects, including beryllium sensitization and the potentially fatal chronic beryllium disease. Efficient methods for monitoring beryllium contamination in workplaces are valuable to help prevent dan... |
|
|
A radioluminescence study of spectral and dose characteristics of common luminophors.
Radiat. Prot. Dosimetry 100(1-4) , 403-6, (2002) Many synthetic materials are used as thermoluminescence dosemeters for the measurement of the absorbed dose from ionising radiation sources. A part of the absorbed energy leads to a prompt luminescence (radioluminescence, abbreviated RL) which dose behaviour ... |
|
|
Solubility and chemistry of materials encountered by beryllium mine and ore extraction workers: relation to risk.
J. Occup. Environ. Med. 53(10) , 1187-93, (2011) Beryllium mine and ore extraction mill workers have low rates of beryllium sensitization and chronic beryllium disease relative to the level of beryllium exposure. The objective was to relate these rates to the solubility and composition of the mine and mill ... |
|
|
Bioavailability of beryllium oxide particles: an in vitro study in the murine J774A.1 macrophage cell line model.
Exp. Lung Res. 31(3) , 341-60, (2005) Beryllium metal and its oxide and alloys are materials of industrial significance with recognized adverse effects on worker health. Currently, the degree of risk associated with exposure to these materials in the workplace is assessed through measurement of b... |
|
|
Preparation, certification and interlaboratory analysis of workplace air filters spiked with high-fired beryllium oxide.
J. Environ. Monit. 14(2) , 391-401, (2012) Occupational sampling and analysis for multiple elements is generally approached using various approved methods from authoritative government sources such as the National Institute for Occupational Safety and Health (NIOSH), the Occupational Safety and Health... |
|
|
Historical analysis of airborne beryllium concentrations at a copper beryllium machining facility (1964-2000).
Ann. Occup. Hyg. 53(4) , 373-82, (2009) Copper beryllium alloys are the most commonly used form of beryllium; however, there have been few studies assessing occupational exposure in facilities that worked exclusively with this alloy versus those where pure metal or beryllium oxide may also have bee... |
|
|
A miniature metal-ceramic x-ray source for spacecraft instrumentation.
Rev. Sci. Instrum. 69(4) , 1893-7, (1998) Definitive mineralogical identification of materials with x-ray diffraction and fluorescence on remote planetary probes requires the development of a rugged miniature x-ray source that complies with the mass, power, thermal, and electrical management constrai... |
|
|
Structure and vibrations of BenOn (n=3-10) clusters.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 67(2) , 350-4, (2007) The structure and harmonic vibrations of Be(n)O(n) (n=3-10) clusters have been investigated using density functional theory. All structures are found to be cumulenic D(nh) rings (equal bonds, alternating angles), with one intense out of plane mode and three i... |
|
|
[BeO-OSL detectors for dose measurements in cell cultures].
Nuklearmedizin. 48(6) , 227-32, (2009) The absorbed dose is an important parameter in experiments involving irradiation of cells in vitro with unsealed radionuclides. Typically, this is estimated with a model calculation, although the results thus obtained cannot be verified. Generally used real-t... |
|
|
Release of beryllium into artificial airway epithelial lining fluid.
Arch. Environ. Occup. Health 67(4) , 219-28, (2012) Inhaled beryllium particles that deposit in the lung airway lining fluid may dissolve and interact with immune-competent cells resulting in sensitization. As such, solubilization of 17 beryllium-containing materials (ore, hydroxide, metal, oxide, alloys, and ... |