N-Benzylhydroxylamine hydrochloride
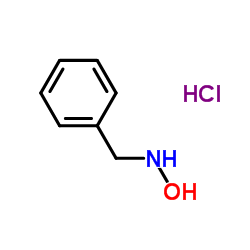
N-Benzylhydroxylamine hydrochloride structure
|
Common Name | N-Benzylhydroxylamine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 29601-98-7 | Molecular Weight | 159.61 | |
| Density | N/A | Boiling Point | 253.9ºC at 760 mmHg | |
| Molecular Formula | C7H10ClNO | Melting Point | 108-110ºC | |
| MSDS | Chinese USA | Flash Point | 135.2ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Regio- and stereoselectivity of captodative olefins in 1,3-dipolar cycloadditions. A DFT/HSAB theory rationale for the observed regiochemistry of nitrones.
J. Org. Chem. 66 , 1252, (2001) Captodative olefins 1-acetylvinyl carboxylates proved to be highly regioselective dipolarophiles in 1,3-dipolar cycloadditon to propionitrile oxide, arylphenylnitrile imines, diazoalkanes, and nitrones to yield the corresponding 5-substituted heterocycles. Th... |
|
|
NBHA reduces acrolein-induced changes in ARPE-19 cells: possible involvement of TGFβ.
Curr. Eye Res. 36(4) , 370-8, (2011) Acrolein, a toxic, reactive aldehyde formed metabolically and environmentally, has been implicated in the damage to and dysfunction of the retinal pigment epithelium (RPE) that accompanies age-related macular degeneration (AMD). Our purpose was to investigate... |
|
|
[1, 3]-Dipolar intramolecular nitrone olefin cycloaddition reaction of a sugar-derived a, ß-unsaturated ester: a new diastereo-and regioselective synthesis of an aminocyclopentitol. Jachak SM, et al.
Tetrahedron Lett. 42(29) , 4925-28, (2001)
|
|
|
The Synthesis of Chiral Polyhydroxylated Pyrrolidines Using a Reverse-Cope Cyclisation. O'Neil IA, et al.
Synlett 10 , 1408-10, (2000)
|
|
|
A Large-Scale Low-Cost Preparation of N-Benzylhydroxylamine Hydrochloride. Nguyen TB, et al.
Synthesis 18 , 3174-76, (2009)
|
|
|
J.E. Baldwin et al.
Tetrahedron 42 , 3097, (1986)
|
|
|
B.J. Wakefield
Sci. Synth. 11 , 229, (2002)
|
|
|
T. Kawakami et al.
Bull. Chem. Soc. Jpn. 73 , 2423, (2000)
|
|
|
S. Murahashi et al.
Tetrahedron Lett. 29 , 2973, (1988)
|
|
|
Ionic liquid mediated synthesis of some novel fluoro isoxazolidine and isoxazoline derivatives using N-benzyl fluoro nitrone via cycloaddition reaction and their antimicrobial activities. Chakraborty B, et al.
Indian J. Chem. B 52(10) , 1342-1351, (2013)
|