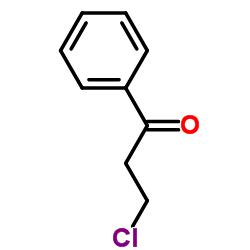
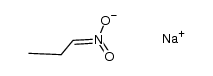
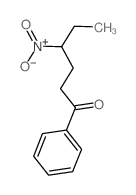
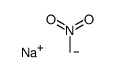
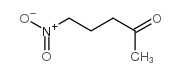
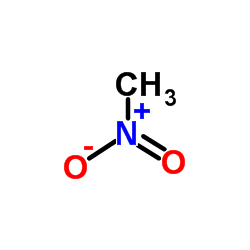
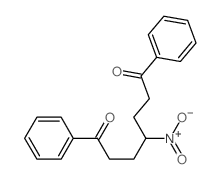
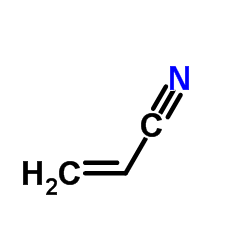
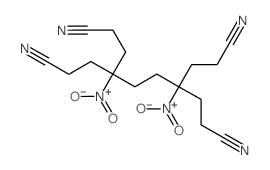
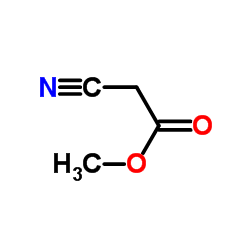
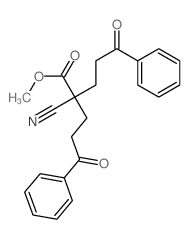
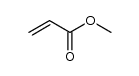
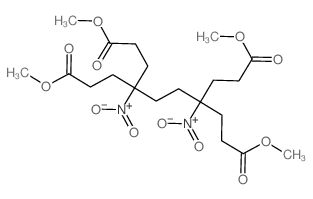
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
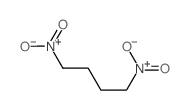

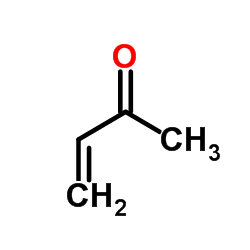
![Ethanone,1,1'-[1,2-ethanediylbis(6-hydroxy-6-methyl-3-nitro-3,1-cyclohexanediyl)]bis- (9CI) Structure](https://image.chemsrc.com/caspic/418/7404-76-4.png)
![1-[5-[2-(3-acetyl-4-methyl-1-nitro-1-cyclohex-3-enyl)ethyl]-2-methyl-5-nitro-1-cyclohexenyl]ethanone Structure](https://image.chemsrc.com/caspic/439/7404-80-0.png)