| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
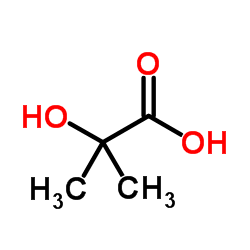 |
2-Hydroxyisobutyric acid
CAS:594-61-6 |
|
 |
(S)-3-Hydroxybutanoic acid
CAS:6168-83-8 |
|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
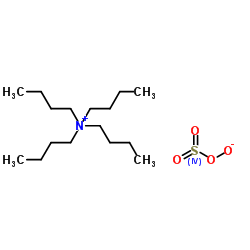 |
Tetrabutylammonium hydrogensulfate
CAS:32503-27-8 |
|
 |
Glycerol
CAS:56-81-5 |
|
 |
Thiophenol
CAS:108-98-5 |
|
 |
Indole-3-butyric acid potassium salt
CAS:60096-23-3 |
|
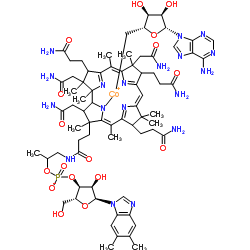 |
coenzyme B12
CAS:13870-90-1 |
|
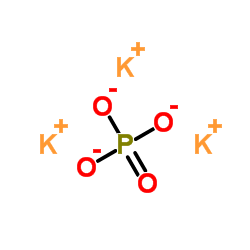 |
Potassium phosphate
CAS:7778-53-2 |