| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
Forskolin
CAS:66575-29-9 |
|
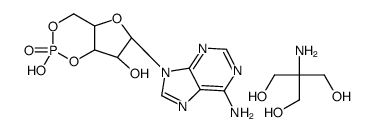 |
ADENOSINE 3':5'-CYCLIC MONOPHOSPHATE TRIS SALT
CAS:102029-77-6 |
|
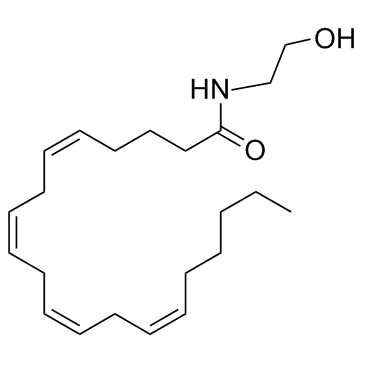 |
Anandamide
CAS:94421-68-8 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
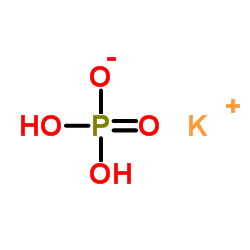 |
Monopotassium phosphate
CAS:7778-77-0 |
|
 |
Pregnenolone
CAS:145-13-1 |
|
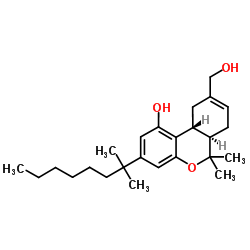 |
hu-210
CAS:112830-95-2 |
|
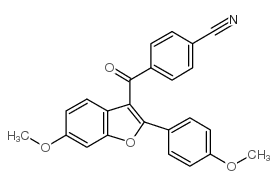 |
LY320135
CAS:176977-56-3 |
|
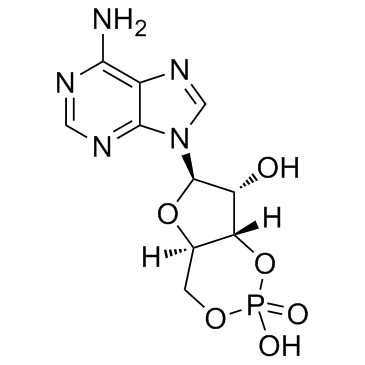 |
Adenosine cyclophosphate
CAS:60-92-4 |