Pregnenolone

Pregnenolone structure
|
Common Name | Pregnenolone | ||
|---|---|---|---|---|
| CAS Number | 145-13-1 | Molecular Weight | 316.478 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 443.3±45.0 °C at 760 mmHg | |
| Molecular Formula | C21H32O2 | Melting Point | 188-190 °C | |
| MSDS | Chinese USA | Flash Point | 188.9±21.3 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of PregnenolonePregnenolone acts as a signaling-specific inhibitor of cannabinoid CB1 receptor, reduces several effects of tetrahydrocannabinol (THC). |
| Name | pregnenolone |
|---|---|
| Synonym | More Synonyms |
| Description | Pregnenolone acts as a signaling-specific inhibitor of cannabinoid CB1 receptor, reduces several effects of tetrahydrocannabinol (THC). |
|---|---|
| Related Catalog | |
| Target |
CB1 Receptor Human Endogenous Metabolite |
| In Vitro | The effect of THC is significantly attenuated when slices are pre-treated with Pregnenolone 100 nM (15.1±1.8 % of inhibition). These effects are likely due to a pre-synaptic action of Pregnenolone. Thus, Pregnenolone blocks the increase in paired-pulse ratio (PPR) induced by THC but does not modify either the amplitude or the decay time of miniature EPSC (mEPSC)[1]. |
| In Vivo | Pregnenolone administration (2-6 mg/kg) blocks THC-induced food-intake in Wistar rats and in C57BL/6N mice, and blunts the memory impairment induced by THC in mice, but it does not modify these behaviors per se. Injections of Pregnenolone (2 and 4mg/kg) before each self-administration session reduce the intake of WIN 55,212-2 and reduce the break-point in a progressive ratio schedule[1]. |
| Animal Admin | Mice and Rats[1] Adult male Wistar rats (weighing 320-340g), Sprague Dawley male rats (weighing 330-350g), C57BL/6N mice (2-3 months) and CD1 mice (weighing 25-30 g at the beginning of the experiments) are used. Pregnenolone is injected subcutaneously (sc). The injection volumes are 1 mL/kg of body weight for rats and 10 mL/kg for mice[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 443.3±45.0 °C at 760 mmHg |
| Melting Point | 188-190 °C |
| Molecular Formula | C21H32O2 |
| Molecular Weight | 316.478 |
| Flash Point | 188.9±21.3 °C |
| Exact Mass | 316.240234 |
| PSA | 37.30000 |
| LogP | 4.52 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.550 |
| InChIKey | ORNBQBCIOKFOEO-UHFFFAOYSA-N |
| SMILES | CC(=O)C1CCC2C3CC=C4CC(O)CCC4(C)C3CCC12C |
| Storage condition | 2~8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302 + H312 + H332-H319 |
| Precautionary Statements | P210-P280-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xi |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TU5560700 |
| HS Code | 2937290090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2937290090 |
|---|
|
Mechanistic Scrutiny Identifies a Kinetic Role for Cytochrome b5 Regulation of Human Cytochrome P450c17 (CYP17A1, P450 17A1).
PLoS ONE 10 , e0141252, (2015) Cytochrome P450c17 (P450 17A1, CYP17A1) is a critical enzyme in the synthesis of androgens and is now a target enzyme for the treatment of prostate cancer. Cytochrome P450c17 can exhibit either one or... |
|
|
HBCDD-induced sustained reduction in mitochondrial membrane potential, ATP and steroidogenesis in peripubertal rat Leydig cells.
Toxicol. Appl. Pharmacol. 282(1) , 20-9, (2015) Hexabromocyclododecane (HBCDD), a brominated flame retardant added to various consumer products, is a ubiquitous environmental contaminant. We have previously shown that 6-hour exposure to HBCDD distu... |
|
|
Calculating virtual log P in the alkane/water system (log P(N)(alk)) and its derived parameters deltalog P(N)(oct-alk) and log D(pH)(alk).
J. Med. Chem. 48 , 3269-79, (2005) Growing interest in the use of both the logarithm of the partition coefficient of the neutral species in the alkane/water system (log P(N)(alk)) and the difference between log P(N)(oct) (the logarithm... |
| EINECS 205-647-4 |
| prenolon |
| ZK-5553 |
| MFCD00003628 |
| Pregn-5-en-20-one, 3-hydroxy- |
| prenenolone |
| Pregnelone |
| Pregnenlolone |
| 3-Hydroxypregn-5-en-20-one |
| Pregnenolone |
| 3β-Hydroxy-5-pregnen-20-one |
| 5-Pregnen-3β-ol-20-one |
| pregnolon |
| Progeserone |
 CAS#:1778-02-5
CAS#:1778-02-5 CAS#:18843-28-2
CAS#:18843-28-2 CAS#:979-02-2
CAS#:979-02-2 CAS#:136634-01-0
CAS#:136634-01-0 CAS#:13057-17-5
CAS#:13057-17-5 CAS#:73465-46-0
CAS#:73465-46-0 CAS#:2722-98-7
CAS#:2722-98-7 CAS#:1162-53-4
CAS#:1162-53-4 CAS#:566-78-9
CAS#:566-78-9 CAS#:387-79-1
CAS#:387-79-1 CAS#:53-43-0
CAS#:53-43-0 CAS#:521-17-5
CAS#:521-17-5![(3S,8R,9S,10R,13S,14S)-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15-decahydr o-1H-cyclopenta[a]phenanthren-3-ol structure](https://image.chemsrc.com/caspic/460/1224-94-8.png) CAS#:1224-94-8
CAS#:1224-94-8 CAS#:901-57-5
CAS#:901-57-5 CAS#:520-88-7
CAS#:520-88-7 CAS#:14148-09-5
CAS#:14148-09-5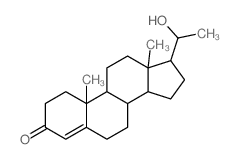 CAS#:145-14-2
CAS#:145-14-2
