| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Crotonic acid
CAS:107-93-7 |
|
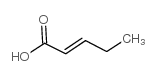 |
trans-2-pentenoic acid
CAS:13991-37-2 |
|
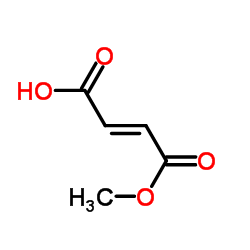 |
Monomethyl fumarate
CAS:2756-87-8 |
|
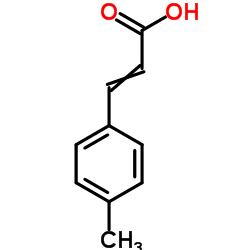 |
4-Methylcinnamic acid
CAS:1866-39-3 |
|
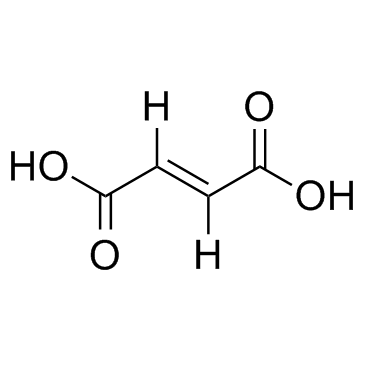 |
fumaric acid
CAS:110-17-8 |
|
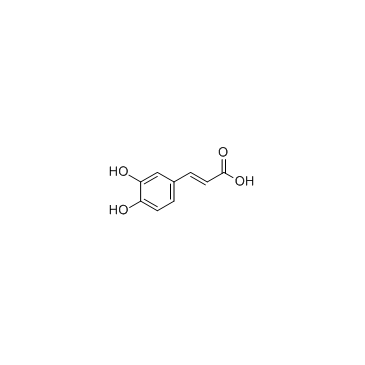 |
Caffeic acid
CAS:331-39-5 |
|
 |
3,3-Dimethylacrylic acid
CAS:541-47-9 |
|
 |
trans-4-Hydroxycinnamic acid
CAS:501-98-4 |
|
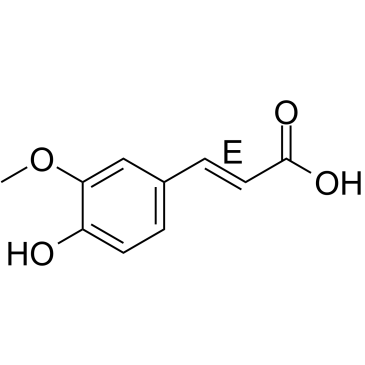 |
(E)-Ferulic acid
CAS:537-98-4 |
|
 |
Cinnamic acid
CAS:140-10-3 |