| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
dichloroethane
CAS:107-06-2 |
|
 |
Sodium deoxycholate
CAS:302-95-4 |
|
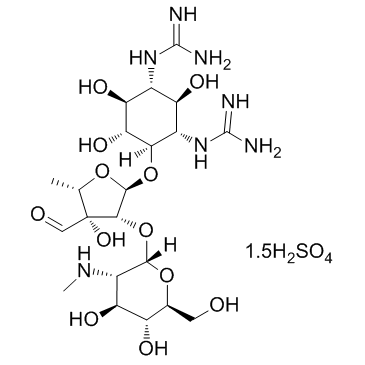 |
Steptomycin sulfate
CAS:3810-74-0 |
|
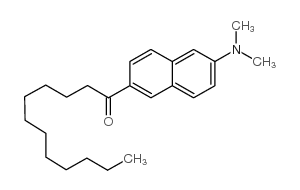 |
Laurdan
CAS:74515-25-6 |
|
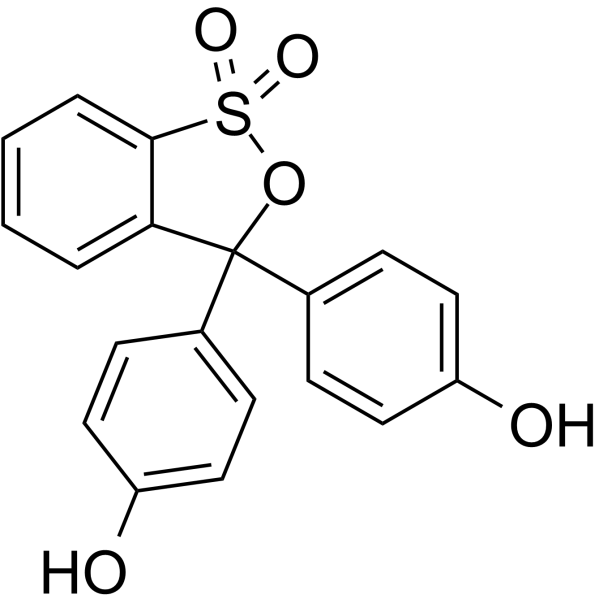 |
Phenol red
CAS:143-74-8 |
|
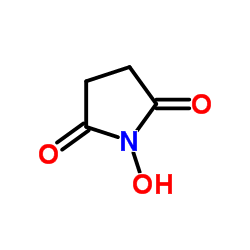 |
N-Hydroxysuccinimide
CAS:6066-82-6 |
|
 |
Fluorescein Diacetate
CAS:596-09-8 |