| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
1-Anthracenamine
CAS:610-49-1 |
|
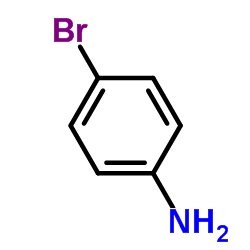 |
4-Bromoaniline
CAS:106-40-1 |
|
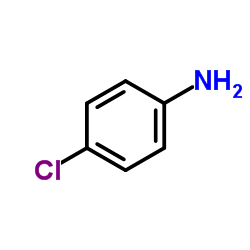 |
4-Chloroaniline
CAS:106-47-8 |
|
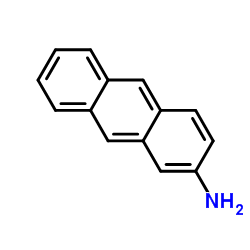 |
2-Anthracenamine
CAS:613-13-8 |
|
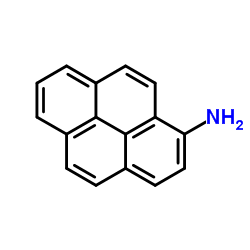 |
aminopyrene
CAS:1606-67-3 |
|
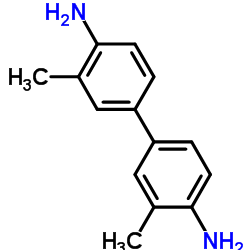 |
3,3'-dimethylbenzidine
CAS:119-93-7 |
|
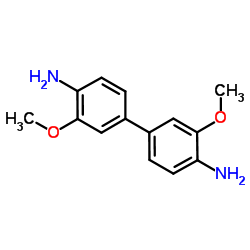 |
3,3'-Dimethoxybiphenyl-4,4'-diamine
CAS:119-90-4 |
|
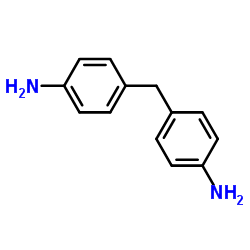 |
4,4′-methylenedianiline
CAS:101-77-9 |
|
 |
4-Phenoxyaniline
CAS:139-59-3 |
|
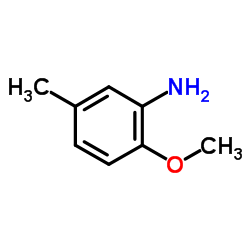 |
Para-Cresidine
CAS:120-71-8 |