| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
L-(+)-Lysine monohydrochloride
CAS:657-27-2 |
|
 |
L-Glutamine
CAS:56-85-9 |
|
 |
Vitamin E
CAS:10191-41-0 |
|
 |
HEPES
CAS:7365-45-9 |
|
 |
12-O-tetradecanoylphorbol-13-acetate
CAS:16561-29-8 |
|
 |
2-Thiophenecarbohydrazide
CAS:2361-27-5 |
|
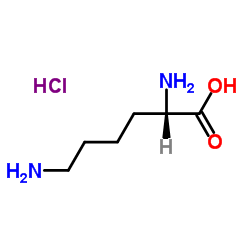 |
L-Lysine hydrochloride
CAS:10098-89-2 |
|
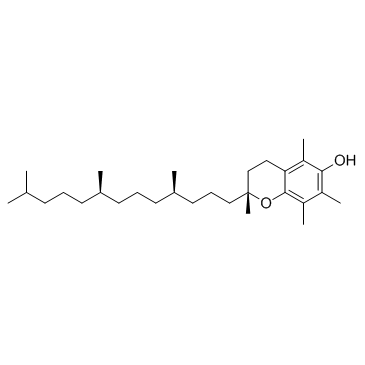 |
DL-alpha-Tocopherol
CAS:59-02-9 |
|
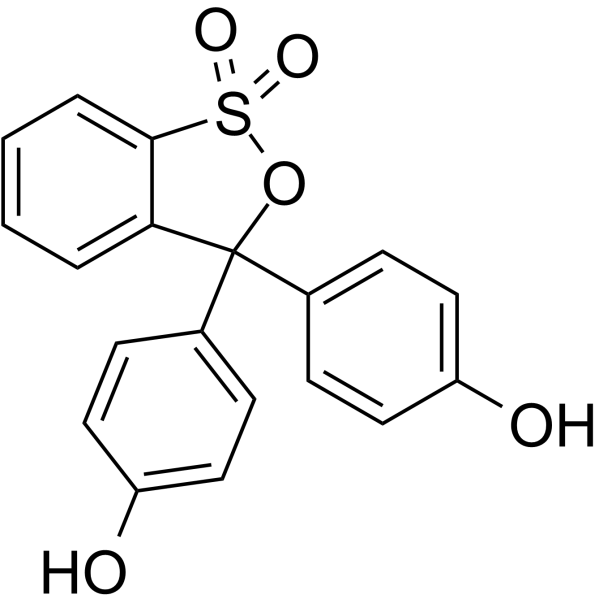 |
Phenol red
CAS:143-74-8 |