Fluorinated phenylcyclopropylamines. Part 4: effects of aryl substituents and stereochemistry on the inhibition of monoamine oxidases by 1-aryl-2-fluoro-cyclopropylamines.
Song Ye, Shinichi Yoshida, Roland Fröhlich, Günter Haufe, Kenneth L Kirk
Index: Bioorg. Med. Chem. 13(7) , 2489-99, (2005)
Full Text: HTML
Abstract
A series of para-ring-substituted (E)- and (Z)-1-aryl-2-fluorocyclopropylamines were examined as inhibitors of recombinant human liver monoamine oxidase A (MAO A) and B (MAO B). Unlike the parent 1-phenylcyclopropylamine, which is a selective inhibitor of MAO B, both (E)- and (Z)-diastereomers of derivatives having fluorine at the 2-position of the cyclopropane ring were potent and selective irreversible inhibitors of MAO A. Both electron releasing groups (Me, OMe) and electron attracting groups (Cl, F) substituted in the para-position caused a modest increase in activity. Geminal difluoro-substitution caused a loss of potency of 100-fold compared to either (E)- or (Z)-monofluorinated analogue. Surprisingly, (1S,2R)-2-fluoro-1-phenylcyclopropylamine and the (1R,2S)-enantiomer were essential equally potent as inhibitors of MAO A and MAO B. None of the tested 1-aryl-2-fluorocyclopropylamines exhibited significant inhibition of tyramine oxidase.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
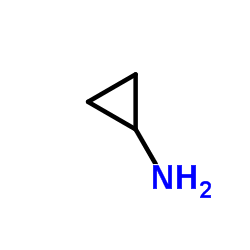 |
Cyclopropanamine
CAS:765-30-0 |
C3H7N |
|
Synthesis and antibacterial activity of some novel 4-oxopyri...
2014-11-01 [Arch. Pharm. (Weinheim) 347(11) , 861-72, (2014)] |
|
Cyclopropylamines from N,N-dialkylcarboxamides and Grignard ...
2010-12-10 [Chemistry 16 , 13862-13875, (2010)] |
|
Cyclopropylamine inactivation of cytochromes P450: role of m...
2005-04-15 [Arch. Biochem. Biophys. 436(2) , 265-75, (2005)] |
|
The Kulinkovich reaction in the synthesis of constrained n,n...
2007-05-10 [Org. Lett. 9(10) , 1987-90, (2007)] |
|
Mutation of surface cysteine 374 to alanine in monoamine oxi...
2005-05-16 [Bioorg. Med. Chem. 13(10) , 3487-95, (2005)] |