| Structure | Name/CAS No. | Articles |
|---|---|---|
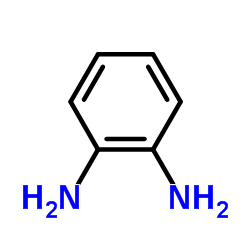 |
o-Phenylenediamine
CAS:95-54-5 |
|
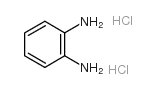 |
1,2-Benzenediamine,hydrochloride (1:2)
CAS:615-28-1 |
|
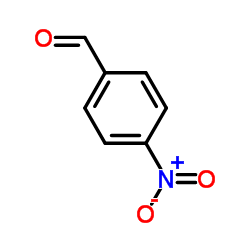 |
4-Nitrobenzaldehyde
CAS:555-16-8 |
|
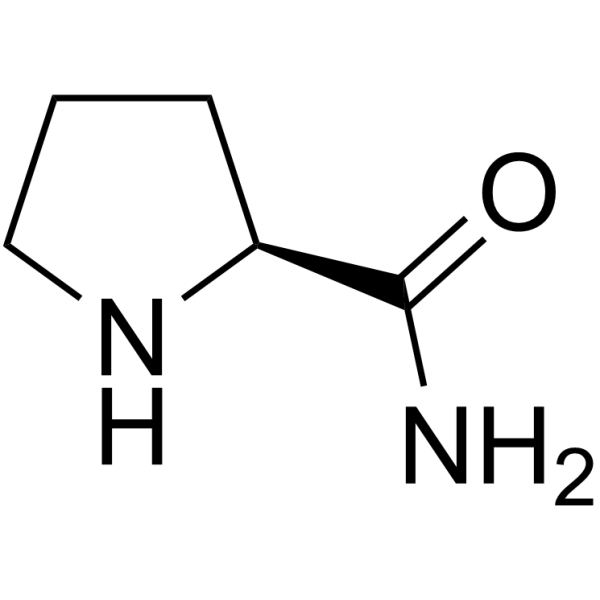 |
L-Prolinamide
CAS:7531-52-4 |