| Structure | Name/CAS No. | Articles |
|---|---|---|
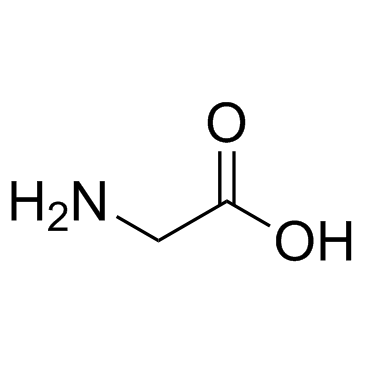 |
Glycine
CAS:56-40-6 |
|
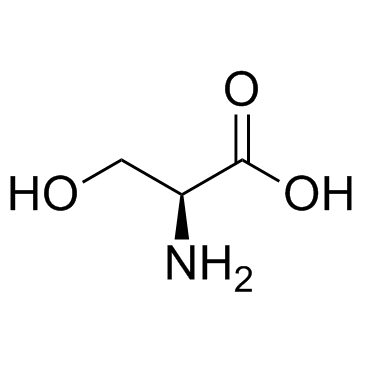 |
L-serine
CAS:56-45-1 |
|
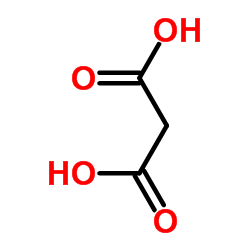 |
Malonic acid
CAS:141-82-2 |
|
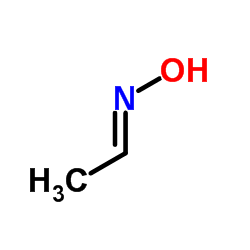 |
Acetaldoxime
CAS:107-29-9 |
|
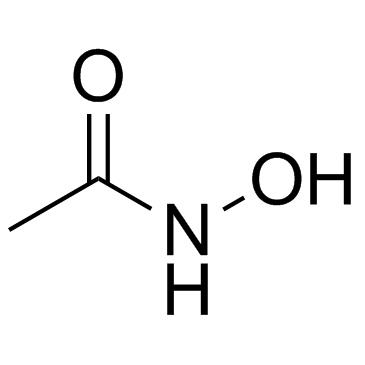 |
Acetohydroxamic acid
CAS:546-88-3 |
|
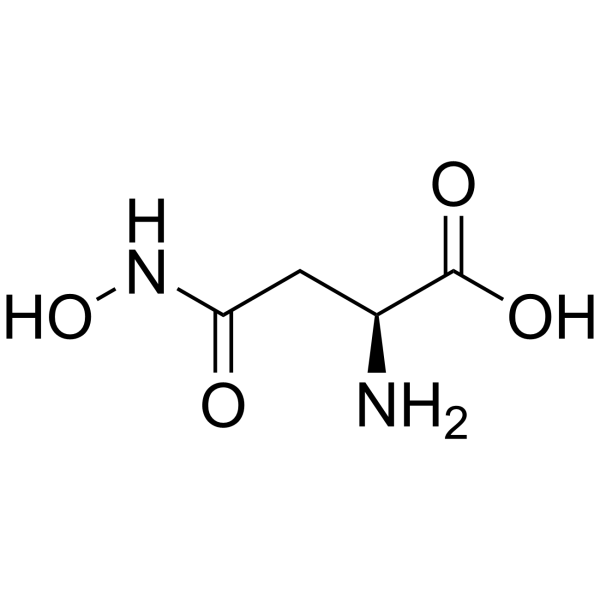 |
L-Asparagine,N-hydroxy
CAS:1955-68-6 |