| Structure | Name/CAS No. | Articles |
|---|---|---|
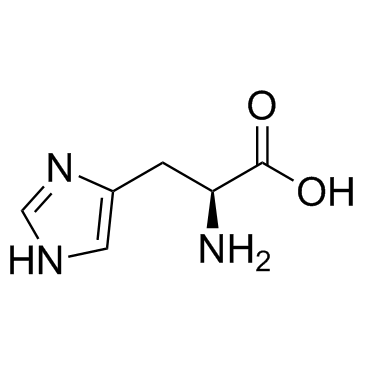 |
L-Histidine
CAS:71-00-1 |
|
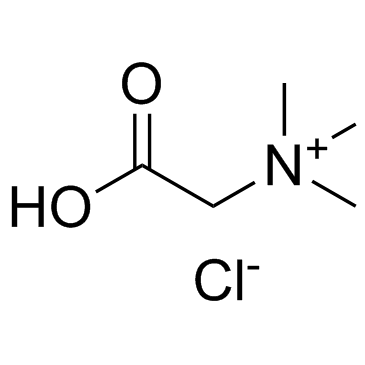 |
Betaine Hydrochloride
CAS:590-46-5 |
|
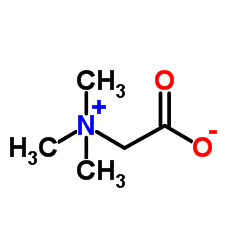 |
Betaine
CAS:107-43-7 |
|
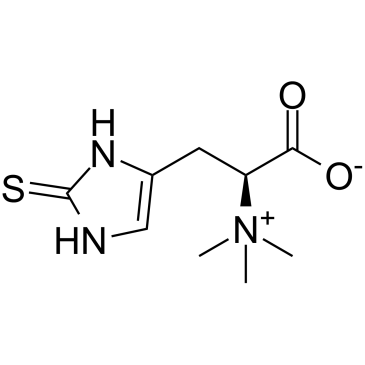 |
L-(+)-Ergothioneine
CAS:497-30-3 |