| Structure | Name/CAS No. | Articles |
|---|---|---|
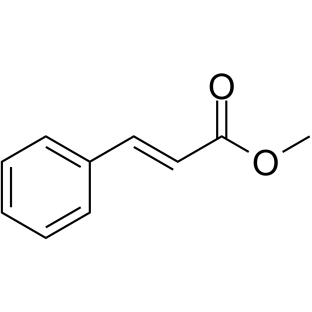 |
Methyl cinnamate
CAS:103-26-4 |
|
 |
Chloramine-T hydrate
CAS:149358-73-6 |
|
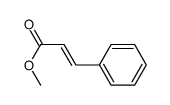 |
methyl (E)-cinnamate
CAS:1754-62-7 |
|
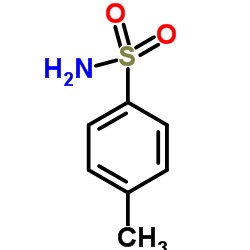 |
4-Toluenesulfonamide
CAS:70-55-3 |
|
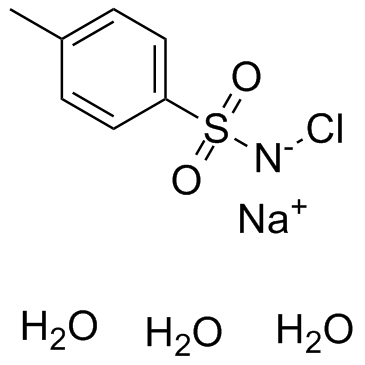 |
Chloramine-T trihydrate
CAS:7080-50-4 |