Chloramine-T trihydrate
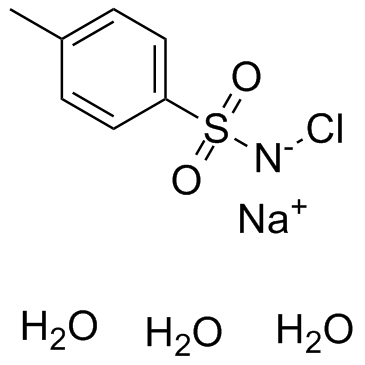
Chloramine-T trihydrate structure
|
Common Name | Chloramine-T trihydrate | ||
|---|---|---|---|---|
| CAS Number | 7080-50-4 | Molecular Weight | 281.690 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C7H13ClNNaO5S | Melting Point | 167-170 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 92 °C | |
| Symbol |



GHS05, GHS07, GHS08 |
Signal Word | Danger | |
|
Catalytic diastereoselective sulfimidation of diaryl sulfides and application of chiral sulfimides to asymmetric allylic alkylation.
Chirality 12(5-6) , 299-312, (2000) The copper-catalyzed diastereoselective imidation of diaryl sulfides bearing a chiral oxazolinyl moiety at the ortho-position with [N-(p-toluenesulfonyl) imino]phenyliodinane (TsN=IPh) or Chloramine-T trihydrate [TsN(Cl)Na.3H2O] was successfully carried out t... |
|
|
The estimation of microgram amounts of methionine by reaction with chloramine-T.
Anal. Biochem. 93 , 419, (1979)
|
|
|
THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY.
Biochem. J. 89 , 114, (1963)
|
|
|
Catalytic asymmetric aminohydroxylation provides a short taxol side-chain synthesis.
Acta Chem. Scand. 50 , 649, (1996) The p-toluenesulfonamide derivate of the C-13 side-chain of taxol was prepared on a one third mole scale in a single step from methyl cinnamate. The process employed is catalytic asymmetric aminohydroxylation (catalytic AA). In the present case, there is no w... |
|
|
Microwave-assisted synthesis of 3,5-disubstituted isoxazoles and evaluation of their anti-ageing activity.
Eur. J. Med. Chem. 83 , 508-15, (2014) One-pot uncatalysed microwave-assisted 1,3-dipolar cycloaddition reactions between in situ generated nitrile oxides and alkynes bearing protected antioxidant substituents, were regioselectively afforded 3,5-disubstituted isoxazoles. The yields were moderate, ... |
|
|
Silica-water reaction media: its application to the formation and ring opening of aziridines.
Angew. Chem. Int. Ed. Engl. 43 , 79, (2004)
|
|
|
Heteropoly acid as a novel nitrene transfer agent: a facile and practical aziridination of olefins with Chloramine-T.
Chem. Commun. (Camb.) , 1026, (2004) Environmentally benign HPA is found to be an efficient catalyst for aziridination of olefins in the presence of inexpensive Chloramine-T as a nitrogen source: instantaneous at room temperature, requires only stoichiometric amount of olefin and no allyl amine ... |
|
|
Tolerability and efficacy of N-chlorotaurine in comparison with chloramine T for the treatment of chronic leg ulcers with a purulent coating: a randomized phase II study.
Br. J. Dermatol. 149(3) , 590-7, (2003) The well-known active chlorine compound chloramine T (CAT) with broad-spectrum antimicrobial activity is in common therapeutic use for leg ulcers with purulent coatings; however, this treatment is painful. The tolerability of the less aggressive N-chlorotauri... |
|
|
Mogilaiah, K.; Reddy, G. R.
J. Chem. Res. (M) , 145, (2004)
|
|
|
Pal, A. et al
Tetrahedron 55 , 4123, (1999)
|