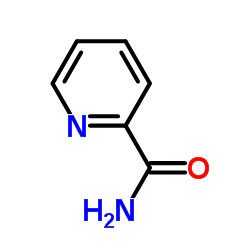
1452-77-3
| Name | picolinamide |
|---|---|
| Synonyms |
pyridine-2-carboxamide
Picolinamide 2-Pyridinecarboxamide 2-Pyridinecarboximidic acid 2-PicolinaMide EINECS 215-921-5 MFCD00023483 |
| Description | Picolinamide (2-Picolinamide) is an inhibitor of Poly(ADP-ribose) synthetase of nuclei from rat pancreatic islet cells[1][3]. |
|---|---|
| Related Catalog | |
| Target |
Poly(ADP-ribose) synthetase[1] |
| In Vitro | Picolinamide (10 μM-1 mM) inhibits Poly(ADP-ribose) synthetase activity[2]. Picolinamide (2 mM) protects against streptozotocin-induced depression of proinsulin synthesis in isolated pancreatic islets of rats[3]. |
| In Vivo | Picolinamide (4 mmol/kg, i.p., rats) inhibits Na+/phosphate cotransport by isolated renal brush border membrane vesicles[1]. Picolinamide (250 mg/kg, i.p., rats) enhances the tumorigenic effect of Streptozotocin and Alloxan on islet B-cells[4]. Animal Model: Rats[1] Dosage: 4 mmol/kg Administration: Intraperitoneal injection (i.p.) Result: Increased renal cortical NAD content (1.5 fold). |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 257.7±32.0 °C at 760 mmHg |
| Melting Point | 110 °C (dec.)(lit.) |
| Molecular Formula | C6H6N2O |
| Molecular Weight | 122.125 |
| Flash Point | 109.7±25.1 °C |
| Exact Mass | 122.048012 |
| PSA | 55.98000 |
| LogP | -0.24 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.590 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933399090 |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |