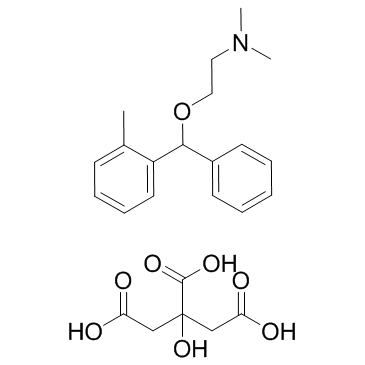
4682-36-4
| Name | orphenadrine citrate |
|---|---|
| Synonyms |
Orphenadrine Citrate
Orphenadrine Citrate Salt MFCD00079197 EINECS 225-137-5 |
| Description | Orphenadrine citrate is a NMDA receptor antagonist with Ki of 6.0 +/- 0.7 μM, HERG potassium channel blocker.Target: NMDA ReceptorOrphenadrine has been used as an antiparkinsonian, antispastic and analgesic drug. Orphenadrine inhibits [3H]MK-801 binding to the phencyclidine (PCP) binding site of the N-methyl-D-aspartate (NMDA)-receptor in homogenates of postmortem human frontal cortex with a Ki-value of 6.0 +/- 0.7 microM. The NMDA receptor antagonistic effects of orphenadrine were assessed using concentration- and patch-clamp techniques on cultured superior colliculus neurones. Orphenadrine blocked open NMDA receptor channels with fast kinetics and in a strongly voltage-dependent manner. The IC50-value against steady state currents at -70 mV was 16.2 +/- 1.6 microM (n = 6). Orphenadrine exhibited relatively fast, concentration-dependent open channel blocking kinetics (Kon 0.013 +/- 0.002 10(6) M-1S-1) whereas the offset rate was concentration-independent (Koff 0.230 +/- 0.004 S-1) [1]. Orphenadrine competitively inhibited [3H]nisoxetine binding in rat vas deferens membranes (Ki = 1.05+/-0.20 microM). It can be concluded that orphenadrine, at low micromolar concentrations, interacts with the noradrenaline reuptake system inhibiting its functionality and thus potentiating the effect of noradrenaline [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.014 g/cm3 |
|---|---|
| Boiling Point | 363ºC at 760 mmHg |
| Melting Point | 132-134ºC |
| Molecular Formula | C24H31NO8 |
| Molecular Weight | 461.50500 |
| Flash Point | 107.1ºC |
| Exact Mass | 461.20500 |
| PSA | 133.60000 |
| LogP | 2.75980 |
| Storage condition | 2-8°C |
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H351 |
| Precautionary Statements | P281 |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R22;R40 |
| Safety Phrases | S22-S36 |
| RIDADR | UN 3249 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |