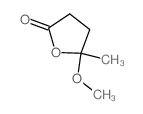
591-12-8
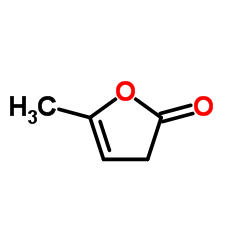
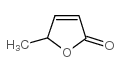
| Name | α-angelica lactone |
|---|---|
| Synonyms |
D2-Angelica lactone
δ2-Angelica lactone EINECS 209-701-8 5-methyl-3H-furan-2-one α-Angelica lactone 5-Methylfuran-2(3H)-one g-Methyl-b,g-crotonolactone alpha-Angelica lactone MFCD00005375 5-Methyl-2(3H)-furanone 4-Hydroxy-3-pentenoic acid γ-lactone a-Angelic lactone a-Angelica Lactone 4-Hydroxy-3-pentenoic acid g-lactone |
| Description | α-Angelica lactone is a naturally occurring anticarcinogen and an vinylogous nucleophile. α-Angelica lactone can give the chiral δ-amino γ,γ-disubstituted butenolide carbonyl derivatives and exhibitselectrophilic trapping at the γ-carbon. α-Angelica lactone exerts strong chemoprotective effects by selective enhancement of glutathione-S-thansferase (GST) and UDP-glucononosyltransferase (UGT) detoxification enzymes[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
Glutathione-S-thansferase (GST) detoxification enzyme[1][2] UDP-glucononosyltransferase (UGT) detoxification enzyme[2] |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 249.4±9.0 °C at 760 mmHg |
| Melting Point | 13-17 °C(lit.) |
| Molecular Formula | C5H6O2 |
| Molecular Weight | 98.100 |
| Flash Point | 68.3±0.0 °C |
| Exact Mass | 98.036781 |
| PSA | 26.30000 |
| LogP | 0.84 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.472 |
| Water Solubility | 5 g/100 mL (25 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| Hazard Codes | Xi |
| Safety Phrases | S24/25 |
| RIDADR | NA 1993 / PGIII |
| WGK Germany | 2 |
| RTECS | LU5075000 |
| HS Code | 2932209090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2932209090 |
|---|---|
| Summary | 2932209090. other lactones. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |