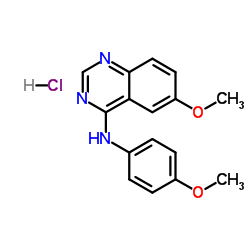
338736-46-2
| Name | 6-Methoxy-N-(4-methoxyphenyl)-4-quinazolinamine hydrochloride (1: 1) |
|---|---|
| Synonyms |
6-Methoxy-N-(4-methoxyphenyl)-4-quinazolinamine hydrochloride (1:1)
(4-methoxy-phenyl)-(6-methoxy-quinazolin-4-yl)-amine HCl 6-Methoxy-N-(4-methoxyphenyl)quinazolin-4-amine hydrochloride (1:1) 4-Quinazolinamine, 6-methoxy-N-(4-methoxyphenyl)-, hydrochloride (1:1) |
| Description | LY456236 is a selective, non-competitive and orally active mGlu1 receptor antagonist that inhibits phosphoinositide hydrolysis with an IC50 of 0.145 μM. LY456236 also inhibits EGFR with an IC50 of 0.91 μM[1][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.145 μM (mGlu1), 0.91 μM (EGFR)[1] |
| In Vitro | LY456236 (2 μM; 30 min) 降低 DHPG (HY-12598A) 刺激的 OCCM-30 增殖[2]。 Cell Proliferation Assay[2] Cell Line: OCCM-30 cells Concentration: 2 μM Incubation Time: 30 min, followed by 72 h incubation with DHPG (HY-12598A) Result: Reduced DHPG-stimulated OCCM-30 proliferation. |
| In Vivo | LY456236 在小鼠 (3-100 mg/kg; i.p.; once) 和大鼠 (10-60 mg/kg; oral; once) 中显示抗惊厥作用。 Animal Model: DBA/2 mice and CD1 mice, seizure models[3] Dosage: 3-100 mg/kg Administration: IP, once Result: Produced dose-related anticonvulsant effects in preventing audiogenic-induced (tonic-clonic) seizures in DBA/2 mice, threshold electroshock-induced seizures in CD1 mice, and 6 Hz electroshock-induced seizures in CD1 mice. Animal Model: Amygdala-kindled Sprague-Dawley rats[3] Dosage: 10, 30 and 60 mg/kg Administration: Oral, once Result: Produced dose-related decreases in behavioral and electrographic seizures at threshold stimulus intensity. Produced a dose-related increase in the stimulus intensity required to produce generalized seizures. |
| References |
| Molecular Formula | C16H16ClN3O2 |
|---|---|
| Molecular Weight | 317.770 |
| Exact Mass | 317.093109 |
| PSA | 56.27000 |
| LogP | 4.26560 |
| Storage condition | 2-8°C |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H318-H335-H413 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | 37/38-41 |
| Safety Phrases | 26-39 |
| RIDADR | NONH for all modes of transport |