71125-38-7
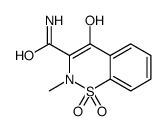
| Name | meloxicam |
|---|---|
| Synonyms |
Meloxicam
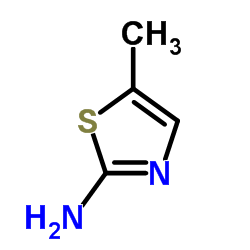
Metacam Parocin 2H-1,2-Benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-, 1,1-dioxide 4-Hydroxy-2-methyl-N-(5-methyl-1,3-thiazol-2-yl)-2H-1,2-benzothiazine-3-carboximidic acid 1,1-dioxide Meloxciam 4-hydroxy-2-méthyl-N-(5-méthyl-1,3-thiazol-2-yl)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxyde 2H-1,2-Benzothiazine-3-carboximidic acid, 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-, 1,1-dioxide Movatec structure was taken from Chemical Abstracts Index Guide, number 4-Hydroxy-2-methyl-N-(5-methyl-1,3-thiazol-2-yl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide meloxicam (tautomer) MOBIC Mobicox MFCD11046047 Movalis MOBEC |
| Description | Meloxicam is a non-steroidal antiinflammatory agent, inhibits COX activity, with IC50s of 0.49 µM and 36.6 µM for COX-2 and COX-1, respectively. |
|---|---|
| Related Catalog | |
| Target |
COX-2:0.49 μM (IC50) COX-1:36.6 μM (IC50) |
| In Vitro | Meloxicam (Compound 5) is a non-steroidal antiinflammatory agent, inhibits COX activity, with IC50s of 0.49 µM and 36.6 µM for COX-2 and COX-1, respectively[1]. Meloxicam inhibits COX+ tumor cells, but shows no cytotoxicity on CF41.Mg or MDCK cells at 0.25-25 µg/mL. Furthermore, Meloxicam in combination with doxorubicin, has no synergistic effect on CF41.Mg cells. Meloxicam (0.25 µg/mL) decreases CF41.Mg cell migration and invasion, induces decrease in MMP-2 expression, and increases β-catenin phophorylation in CF41.Mg cells, but does not affect the CF41.Mg cell apoptosis[2]. |
| In Vivo | Meloxicam (10 mg/kg) alone or in combination with rutin significantly decreases paw liking time on 1st day by 55% and 49% compared with the formalin-treated group, respectively, however the combination reduces time non-significantly on 3rd day in mice. Meloxicam alone or in combination with rutin also decreases relative liver weights, reduces MDA contents, induces liver SOD activities, hampers IL-1β content, and significantly reduces the number of positive caspase-3 immunoreactive cells in mice[3]. |
| Cell Assay | CF41.Mg and MDCK cells are seeded at a density of 1.5 × 103 cells/well into 96-well plates, cultured for 24 h and then exposed to 0-25 µg/mL Meloxicam alone or in combination with doxorubicin. To evaluate synergism and sensitization, doxorubicin is added at the same time and after 24 h, respectively. MDCK cells are exposed only to Meloxicam as a non-tumor negative control. Control groups are cultured without Meloxicam and doxorubicin, but the corresponding amount of DMSO is added to the medium. Following an incubation period of 24 and 48 h, cell growth is measured using the MTS assay, with the absorbance at 490 nm determined using a microplate reader. Each experiment is performed 3 times in triplicate[2]. |
| Animal Admin | Mice[3] Thirty-two mice are randomLy allocated into four groups, eight mice each. Groups are received 20 μL of 2.5% formalin injected in the plantar region of the right hind paw of mice on the 1st and 3rd days 1 h after administration of 1% DMSO (group 1; positive control group), 60 mg/kg rutin, orally (group 2), and oral treatment with 10 mg/kg Meloxicam (group 3), as well as combined treatment of rutin and Meloxicam (group 4). In all groups, drugs are administered once a day for duration of 7 days. On day 7, mice are euthanized and right hind paws plus livers are immediately excised, washed with ice-cold saline, blotted dry, and weighed. They are stored at −80°C till the time of analysis. Then the used animals will be frozen till being incinerated[3]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 581.3±60.0 °C at 760 mmHg |
| Melting Point | 255ºC |
| Molecular Formula | C14H13N3O4S2 |
| Molecular Weight | 351.401 |
| Flash Point | 305.4±32.9 °C |
| Exact Mass | 351.034760 |
| PSA | 136.22000 |
| LogP | 3.35 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.735 |
| Water Solubility | DMSO: soluble |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 26 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | DL0702000 |
| Packaging Group | III |
| HS Code | 2934999090 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |




![Ethyl 4-hydroxy-2-methyl-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide structure](https://image.chemsrc.com/caspic/482/24683-26-9.png)


![N-[(2E)-3,5-Dimethyl-1,3-thiazol-2(3H)-ylidene]-4-hydroxy-2-methy l-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide structure](https://image.chemsrc.com/caspic/266/1145656-36-5.png)
![Sodium 2-methyl-3-[(5-methyl-1,3-thiazol-2-yl)carbamoyl]-2H-1,2-b enzothiazin-4-olate 1,1-dioxide hydrate (1:1:1) structure](https://image.chemsrc.com/caspic/448/71125-39-8.png)