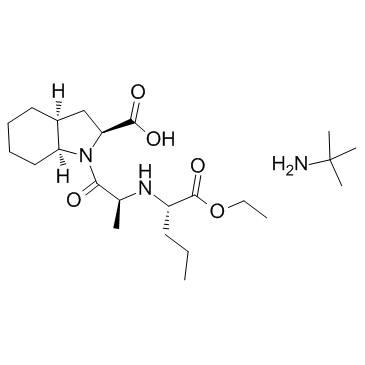
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
NL5999400
-
CHEMICAL NAME :
-
11H-Indole-2-carboxylic acid, octahydro-1-(2-((1-ethoxycarbonyl)butyl)amino)-1-oxop ropyl)-, (2S-(1(R*(R*)),2-alpha,3a-beta,7a-beta))-
-
CAS REGISTRY NUMBER :
-
82834-16-0
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
15
-
MOLECULAR FORMULA :
-
C19-H32-N2-O5
-
MOLECULAR WEIGHT :
-
368.53
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 10,446,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
323 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 10,446,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>2500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - dyspnea Lungs, Thorax, or Respiration - respiratory depression
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 22,1673,1994
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
679 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 10,446,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>1600 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 10,446,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
>500 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 22,1679,1994 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4102 mg/kg/78W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Kidney, Ureter, Bladder - interstitial nephritis Kidney, Ureter, Bladder - changes in bladder weight
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 10,446,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
365 mg/kg/52W-I
-
TOXIC EFFECTS :
-
Cardiac - changes in heart weight Nutritional and Gross Metabolic - weight loss or decreased weight gain Biochemical - Metabolism (Intermediary) - lipids including transport
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 22,1689,1994
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
273 mg/kg/91D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Kidney, Ureter, Bladder - other changes in urine composition Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 10,446,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2184 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Kidney, Ureter, Bladder - interstitial nephritis Kidney, Ureter, Bladder - changes in bladder weight
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 10,446,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
4600 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 22,1711,1994 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
192 mg/kg
-
SEX/DURATION :
-
female 6-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 22,1729,1994
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
416 mg/kg
-
SEX/DURATION :
-
female 17-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 22,1729,1994
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1010 mg/kg
-
SEX/DURATION :
-
male 80 day(s) pre-mating female 2 week(s) pre-mating - 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 22,1729,1994
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
104 mg/kg
-
SEX/DURATION :
-
female 17-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Effects on Newborn - other neonatal measures or effects Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
CDREEA Cardiovascular Drug Reviews. (Raven Press, 1185 Avenue of the Americas, New York, NY 10036) V.6- 1988- Volume(issue)/page/year: 10,446,1992
|
![(2S,3aS,7aS)-1-[(2S)-2-[[(1S)-1-(Ethoxycarbonyl)butyl]amino]-1-oxopropyl]octahydro-1H-indole-2-carboxylic Acid Benzyl Ester structure](https://image.chemsrc.com/caspic/499/122454-52-8.png)
![(2S)-1-{(2S)-2-[(1S)-1-(Ethoxycarbonyl)butylamino]propionyl}-2,3,4,5,6,7-hexahydro-1H-indole-2-carboxylic acid structure](https://image.chemsrc.com/caspic/403/625095-50-3.png)
![(2S,3aS,7aS)-1-{2-[1-ethoxycarbonyl-(S)-butylamino]-(S)-propionyl}-octahydroindole-2-carboxylic acid p-nitrobenzylic ester structure](https://image.chemsrc.com/caspic/432/866430-96-8.png)
![Benzyl (2S)-1-((2S)-2-{[(1S)-1-(ethoxycarbonyl)butyl]amino}-propanoyl)-2-indolinecarboxylate structure](https://image.chemsrc.com/caspic/473/685141-29-1.png)
![ethyl (2S)-2-[(4S)-4-methyl-2-oxido-5-oxo-1,2,3-oxathiazolidin-3-yl]-pentanoate structure](https://image.chemsrc.com/caspic/258/625095-49-0.png)