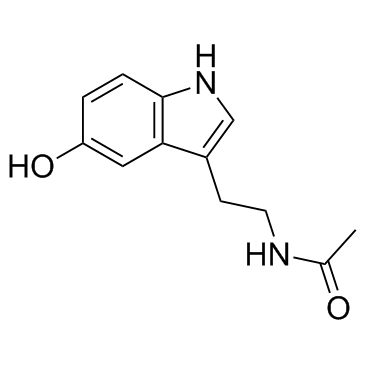
1210-83-9
| Name | N-acetylserotonin |
|---|---|
| Synonyms |
EINECS 214-916-5
N-acetyl-5-HT N-Acetylserotonin 3-(2-Acetamidoethyl)-5-hydroxyindole Normelatonin N-Acetylserotonin,Normelatonin N-Acetylserotonin Normelatonin N-[2-(5-Hydroxy-1H-indol-3-yl)ethyl]acetamide N-Acetyl-5-hydroxytryptamine N-acetyl-serotonin MFCD00005656 5-Hydroxymelatonin 5-Hydroxy-N-acetyltryptamine N-ACETYL SEROTONIN |
| Description | N-Acetyl-5-hydroxytryptamine is a Melatonin precursor, and that it can potently activate TrkB receptor. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite TrkB |
| In Vitro | N-Acetyl-5-hydroxytryptamine (NAS), a precursor of Melatonin, is acetylated from serotonin by AANAT (arylalkylamine N-acetyltransferase). N-acetylserotonin activates TrkB receptor in a circadian rhythm. N-Acetyl-5-hydroxytryptamine swiftly activates TrkB in a circadian manner and exhibits antidepressant effect in a TrkB-dependent manner. N-Acetyl-5-hydroxytryptamine rapidly activates TrkB, but not TrkA or TrkC, in a neurotrophin- and MT3 receptor-independent manner[1]. |
| In Vivo | To explore whether N-Acetyl-5-hydroxytryptamine, can trigger TrkB activation in vivo, TrkB F616A knockin mice are employed, where it has been shown that TrkB F616A activation can be selectively blocked by 1NMPP1, a derivative of kinase inhibitor PP1, leading to TrkB-null phenotypes. To assess whether N-Acetyl-5-hydroxytryptamine can mimic BDNF, cortical neurons from TrkB F616A knockin mice are prepared. In alignment with a previous report, BDNF- and NAS-mediated TrkB phosphorylation are selectively reduced by 1NMPP1 but not by K252a, whereas serotonin or Melatonin had no effect . These findings suggest that NAS strongly provokes both wild-type TrkB and TrkB F616A tyrosine phosphorylation and activation[1]. |
| Animal Admin | Mice[1] Two-month-old TrkB F616A mice are pretreated with 1NMPP1 in drinking water (50 μM) 1 day before the experiment, followed by administration of N-Acetyl-5-hydroxytryptamine (20 mg/kg, i.p.) or Melatonin (1 mg/kg, i.p.). Mice are killed at 1 h. The brain homogenates are analyzed by immunoblotting with anti-p-TrkB. Two- to three-month-old BDNF forebrain conditional knockout mice are injected i.p. with N-Acetyl-5-hydroxytryptamine or Melatonin. Mice are killed at 0, 0.5, 1, or 2 h following drug administration. Brain lysates are prepared and analyzed by immunoblotting with anti-phospho-TrkB Y816[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 556.8±40.0 °C at 760 mmHg |
| Melting Point | 120-122 °C(lit.) |
| Molecular Formula | C12H14N2O2 |
| Molecular Weight | 218.252 |
| Flash Point | 290.6±27.3 °C |
| Exact Mass | 218.105530 |
| PSA | 65.12000 |
| LogP | -0.13 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.651 |
| Storage condition | 2-8°C |
| Water Solubility | ethanol: 50 mg/mL |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

![[3-(2-acetamidoethyl)-1H-indol-5-yl] acetate structure](https://image.chemsrc.com/caspic/469/28026-16-6.png)

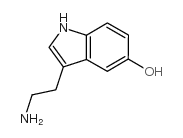


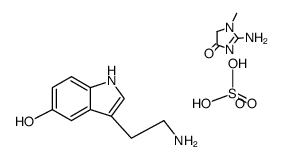
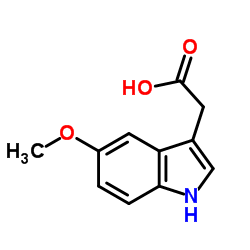
![N-[2-(1-hydroxy-5-methoxyindol-3-yl)ethyl]acetamide structure](https://image.chemsrc.com/caspic/236/180910-62-7.png)