N-Acetylserotonin
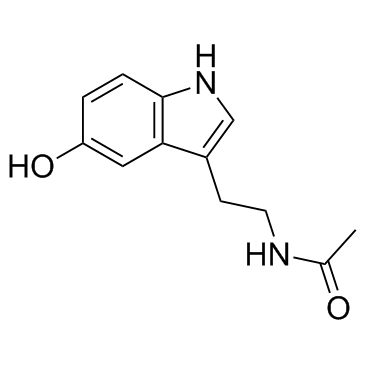
N-Acetylserotonin structure
|
Common Name | N-Acetylserotonin | ||
|---|---|---|---|---|
| CAS Number | 1210-83-9 | Molecular Weight | 218.252 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 556.8±40.0 °C at 760 mmHg | |
| Molecular Formula | C12H14N2O2 | Melting Point | 120-122 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 290.6±27.3 °C | |
Use of N-AcetylserotoninN-Acetyl-5-hydroxytryptamine is a Melatonin precursor, and that it can potently activate TrkB receptor. |
| Name | N-acetylserotonin |
|---|---|
| Synonym | More Synonyms |
| Description | N-Acetyl-5-hydroxytryptamine is a Melatonin precursor, and that it can potently activate TrkB receptor. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite TrkB |
| In Vitro | N-Acetyl-5-hydroxytryptamine (NAS), a precursor of Melatonin, is acetylated from serotonin by AANAT (arylalkylamine N-acetyltransferase). N-acetylserotonin activates TrkB receptor in a circadian rhythm. N-Acetyl-5-hydroxytryptamine swiftly activates TrkB in a circadian manner and exhibits antidepressant effect in a TrkB-dependent manner. N-Acetyl-5-hydroxytryptamine rapidly activates TrkB, but not TrkA or TrkC, in a neurotrophin- and MT3 receptor-independent manner[1]. |
| In Vivo | To explore whether N-Acetyl-5-hydroxytryptamine, can trigger TrkB activation in vivo, TrkB F616A knockin mice are employed, where it has been shown that TrkB F616A activation can be selectively blocked by 1NMPP1, a derivative of kinase inhibitor PP1, leading to TrkB-null phenotypes. To assess whether N-Acetyl-5-hydroxytryptamine can mimic BDNF, cortical neurons from TrkB F616A knockin mice are prepared. In alignment with a previous report, BDNF- and NAS-mediated TrkB phosphorylation are selectively reduced by 1NMPP1 but not by K252a, whereas serotonin or Melatonin had no effect . These findings suggest that NAS strongly provokes both wild-type TrkB and TrkB F616A tyrosine phosphorylation and activation[1]. |
| Animal Admin | Mice[1] Two-month-old TrkB F616A mice are pretreated with 1NMPP1 in drinking water (50 μM) 1 day before the experiment, followed by administration of N-Acetyl-5-hydroxytryptamine (20 mg/kg, i.p.) or Melatonin (1 mg/kg, i.p.). Mice are killed at 1 h. The brain homogenates are analyzed by immunoblotting with anti-p-TrkB. Two- to three-month-old BDNF forebrain conditional knockout mice are injected i.p. with N-Acetyl-5-hydroxytryptamine or Melatonin. Mice are killed at 0, 0.5, 1, or 2 h following drug administration. Brain lysates are prepared and analyzed by immunoblotting with anti-phospho-TrkB Y816[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 556.8±40.0 °C at 760 mmHg |
| Melting Point | 120-122 °C(lit.) |
| Molecular Formula | C12H14N2O2 |
| Molecular Weight | 218.252 |
| Flash Point | 290.6±27.3 °C |
| Exact Mass | 218.105530 |
| PSA | 65.12000 |
| LogP | -0.13 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.651 |
| InChIKey | MVAWJSIDNICKHF-UHFFFAOYSA-N |
| SMILES | CC(=O)NCCc1c[nH]c2ccc(O)cc12 |
| Storage condition | 2-8°C |
| Water Solubility | ethanol: 50 mg/mL |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
The antioxidant behaviour of melatonin and structural analogues during lipid peroxidation depends not only on their functional groups but also on the assay system.
Biochem. Biophys. Res. Commun. 423(4) , 873-7, (2012) There is no general agreement yet on the antioxidant effect of pineal indoles against lipid peroxidation. Accordingly, the main goal of the present work was to study the antioxidant activity of melato... |
|
|
Clock-Controlled Regulation of the Acute Effects of Norepinephrine on Chick Pineal Melatonin Rhythms.
J. Biol. Rhythms 30 , 519-32, (2015) The chicken pineal gland synthesizes and releases melatonin rhythmically in light/dark (LD) cycles, with high melatonin levels during the dark phase, and in constant darkness (DD) for several cycles b... |
| EINECS 214-916-5 |
| N-acetyl-5-HT |
| N-Acetylserotonin |
| 3-(2-Acetamidoethyl)-5-hydroxyindole |
| Serotonin, N-acetyl- |
| Normelatonin |
| N-Acetylserotonin,Normelatonin |
| Acetamide, N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]- |
| N-Acetylserotonin Normelatonin |
| N-[2-(5-Hydroxy-1H-indol-3-yl)ethyl]acetamide |
| N-Acetyl-5-hydroxytryptamine |
| N-acetyl-serotonin |
| MFCD00005656 |
| 5-Hydroxymelatonin |
| 5-Hydroxy-N-acetyltryptamine |
| N-ACETYL SEROTONIN |
 CAS#:68062-88-4
CAS#:68062-88-4 CAS#:73-31-4
CAS#:73-31-4![[3-(2-acetamidoethyl)-1H-indol-5-yl] acetate Structure](https://image.chemsrc.com/caspic/469/28026-16-6.png) CAS#:28026-16-6
CAS#:28026-16-6 CAS#:2436-15-9
CAS#:2436-15-9 CAS#:108-24-7
CAS#:108-24-7 CAS#:20776-45-8
CAS#:20776-45-8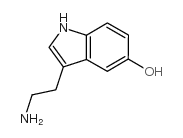 CAS#:50-67-9
CAS#:50-67-9 CAS#:75-36-5
CAS#:75-36-5 CAS#:153-98-0
CAS#:153-98-0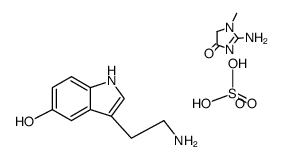 CAS#:971-74-4
CAS#:971-74-4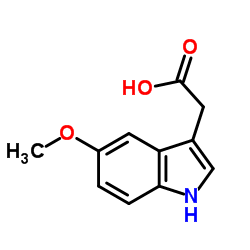 CAS#:608-07-1
CAS#:608-07-1![N-[2-(1-hydroxy-5-methoxyindol-3-yl)ethyl]acetamide structure](https://image.chemsrc.com/caspic/236/180910-62-7.png) CAS#:180910-62-7
CAS#:180910-62-7