127191-97-3
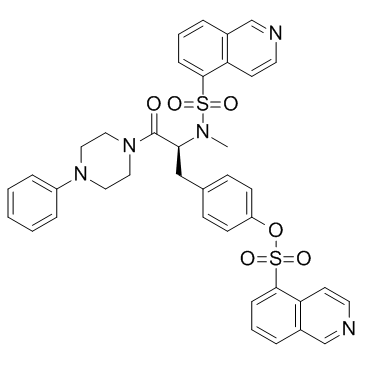
| Name | 4-[(2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl]phenylisoquinolinesulfonicacidester |
|---|---|
| Synonyms |
kn-62
4-[(2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo- 3-(4-phenyl-1-piperazinyl)propyl]phenylisoquinolinesulfonicacid ester KN-62 4-[(2S)-2-[(5-Isoquinolinylsulfonyl)(methyl)amino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl]phenyl 5-isoquinolinesulfonate 1-(N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl)-4-phenylpiperazine 4-[(2S)-2-[(Isoquinolin-5-ylsulfonyl)(methyl)amino]-3-oxo-3-(4-phenylpiperazin-1-yl)propyl]phenyl isoquinoline-5-sulfonate MFCD00083180 5-Isoquinolinesulfonic acid, 4-[(2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl]phenyl ester |
| Description | KN-62 is a selective and potent inhibitor of calmodulin-dependent protein kinase II (CaMK-II) with IC50 of 0.9 μM, KN-62 also displays noncompetitive antagonism at P2X7 receptors in HEK293 cells, with an IC50 value of approximately 15 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.9 μM (CaMK II)[1], 15 nM (P2X7 receptor, in HEK293 cells)[2] |
| In Vitro | KN-62 is a selective antagonist of Ca2+/calmodulin-dependent protein kinase II (CaMKII). KN-62 potently antagonizes ATP-stimulated Ba2+ influx into fura-2 loaded human lymphocytes with an IC50 of 12.7±1.5 nM (n=3) and complete inhibition of the flux at a concentration of 500 nM. Similarly, KN-62 inhibits ATP-stimulated ethidium+ uptake, measured by time resolved flow cytometry, with an IC50 of 13.1±2.6 nM (n=4) and complete inhibition of the flux at 500 nM[1]. KN-62 is found to be a potent antagonist in a functional assay, inhibition of ATP-induced K+efflux in HEK293 cells expressing recombinant human P2X7 receptors. In human leukemic B lymphocytes, KN-62 reduces the rate of permeability increase to larger permeant cations, like ethidium, induced by Bz-ATP with an IC50 of 13.1 nM. KN-62 at a concentration of 3 µM has no effect on ATP-induced ethidium influx through the rat P2X7 receptor, while the IC50 at the human P2X7 receptor is 0.1 µM. KN-62 has considerable selectivity for P2X7 receptors within the P2 family[2]. |
| In Vivo | The antidepressant-like behavior of ZnCl2 (10 mg/kg, p.o.) (p<0.01) is prevented by CAMKII inhibitor KN-62 (1 μg/site, i.c.v.). The two-way ANOVA reveals a significantly main effect of KN-62 treatment [F(1,28)=27.47, p<0.01], no main effect of ZnCl2 treatment [F(1,28)=0.84, p>0.05] and a significant effect of KN-62×ZnCl2 treatment interaction [F(1,28)=22.57, p<0.01] to immobility time. As revealed by the post-hoc analysis, the anti-immobility effect of ZnCl2 is completely prevented by treatment of animals with KN-62. No effect in locomotor activity in the open-field test is observed: (KN-62 treatment [F(1,24)=1.97, p>0.05], ZnCl2 treatment [F(1,24)=3.99, p>0.05] and KN-62×ZnCl2 treatment interaction [F(1,24)=0.61, p>0.05])[3]. |
| Kinase Assay | Lymphocytes (1×107/mL) are cultured with [3H]-oleic acid (2-5 μCi/mL, specific activity 10 Ci/mmol) for 20-24 h in RPMI-1640 medium supplemented with Gentamicin (40 μg/mL), 10% heat inactivated foetal calf serum (FCS) at 37°C to label membrane phospholipids. Labelled cells are washed twice in HEPES buffered saline followed by a final wash in either HEPES buffered saline or 150 mM KCl medium containing HEPES 10 mM, pH 7.4, bovine serum albumin (BSA) 1 g/L and D-glucose 5 mM and CaCl2 1 mM. Three mL aliquots containing 1.1×10< sup>7/mL lymphocytes are warmed to 37°C and incubated with or without KN-62 or KN-04 (1 nM-500 nM) for 5 min, then 900 mL aliquots are added to 100 uL butanol (final concentration 30 mM) for a further 5 min, and stimulated with 1 mM ATP for 15 min with gentle mixing in the continued presence of inhibitor or diluent. The phospholipase D reaction is terminated by addition of 1 mL of 20 mM MgCl2 followed by centrifugation and addition of 1 mL ice cold methanol. Membrane lipids are extracted into chloroform/HCl at 4°C under N2, and separated by silica gel thin layer chromatography (t.l.c.) with the solvent system, ethyl acetate/iso-octane/acetic acid/water (13:2:3:10, v/v) under saturating conditions. Sample spots are located by autoradiography and [3H]-phosphatidylbutanol ([3H]-PBut) spots identified by an authentic standard. [3H]-PBut and [3H]-phospholipid spots are scraped into scintillant fluid (PPO in toluene, 4 g/L) and counted in a liquid scintillation counter. The quantity of [3H]-PBut is presented as a percentage of total 3H labelled-cellular phospholipids. Phospholipase D assays are performed in triplicate[1]. |
| Cell Assay | All experiments are performed using adherent HEK293 cells stably transfected with cDNA encoding the human P2X7 receptor. Adherent cells on 12-well polylysine-coated plates are incubated at 37°C in 1 mL physiological salt solution (125 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 25 mM NaHEPES (pH 7.5), 10 mM D-glucose, 1 mg/mL BSA). Antagonists(e.g., KN-62) are added from 1,000× stock solutions dissolved in DMSO. Cells are preincubated with antagonists (e.g., KN-62) for 15 min prior to stimulation for 10 min with 3 mM ATP (final concentration). Reactions are terminated by rapid aspiration of the extracellular medium in each well. The adherent cells in each well are then extracted overnight with 1 mL 10% HNO3. K+ content in these nitric acid extracts is assayed by atomic absorbance spectrophotometry. Duplicate or triplicate wells are run for all test conditions in each separate experiment[2]. |
| Animal Admin | Mice[3] Female Swiss mice (45-55 days old, weighing 30-45 g) are used. The following drugs are used: ZnCl2 (1 or 10 mg/kg), H-89 (1 μg/site, PKA inhibitor), KN-62 (1 μg/site, CAMKII inhibitor), chelerythrine (1 μg/site, PKC inhibitor), PD98059 (5 μg/site, MAPKK/MEK 1/2 inhibitor), U0126 (5 μg/site, MEK1/2 inhibitor), LY294002 (10 nmol/site, PI3K inhibitor), AR-A014418 (0.001 μg/site, selective GSK-3β inhibitor). ZnCl2 is dissolved in distilled water and administered orally (p.o.). H-89, KN-62, chelerythrine, PD98059, U0126, LY294002, AR-A014418 are dissolved in saline (0.9% NaCl) at a final concentration of 1% dimethyl sulfoxide (DMSO) and administered by intracerebroventricular (i.c.v.) route. The drugs are freshly prepared before treatment and administered in a volume of 10 mL/kg body weight (p.o. route) or 5 μL/site (i.c.v. route). Control animals receive the appropriate vehicle. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 964.7±75.0 °C at 760 mmHg |
| Melting Point | 92-94°C |
| Molecular Formula | C38H35N5O6S2 |
| Molecular Weight | 721.844 |
| Flash Point | 537.3±37.1 °C |
| Exact Mass | 721.202881 |
| PSA | 146.84000 |
| LogP | 5.23 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.686 |
| Storage condition | −20°C |
| Water Solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 0.93 mg/mL |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |