1115-70-4
| Name | Metformin Hydrochloride |
|---|---|
| Synonyms |
Metformin HCl
1,1-Dimethylbiguanide hydrochloride,Metformin 3-(diaminomethylidene)-1,1-dimethylguanidine,hydrochloride Imidodicarbonimidic diamide, N,N-dimethyl-, hydrochloride (1:1) 1,1-Dimethylbiguanide hydrochloride MFCD00012582 N,N-Dimethylimidodicarbonimidic diamide hydrochloride (1:1) N1,N1-Dimethylbiguanide hydrochloride Metformin hydrochloride EINECS 214-230-6 Metformin (hydrochloride) |
| Description | Metformin (hydrochloride) is an FDA approved first-line drug for the treatment of type 2 diabetes. Metformin decreases hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory-chain complex 1. |
|---|---|
| Related Catalog | |
| Target |
AMPK Autophagy Mitophagy |
| In Vitro | Metformin inhibits proliferation of ESCs in a concentration-dependent manner. The IC50 is 2.45 mM for A-ESCs and 7.87 mM for N-ESCs. Metformin shows pronounced effects on activation of AMPK signaling in A-ESCs from secretory phase than in cells from proliferative phase[3]. Metformin (0-500 μM) decreases glycogen synthesis in a dose-dependent manner with an IC50 value of 196.5 μM in cultured rat hepatocytes[4]. Metformin shows cell viability and cytotoxic effects on PC-3 cells with IC50 of 5 mM[5]. |
| In Vivo | Metformin (100 mg/kg, p.o.) alone, and metformin (25, 50, 100 mg/kg) with isoproterenol groups attenuates myocyte necrosis through histopathological analysis[1]. Metformin (> 900 mg/kg/day, p.o.) results in moribundity/mortality and clinical signs of toxicity in Crl:CD(SD) rats[2]. |
| Cell Assay | ESCs are plated in 96-well plates at a concentration of 1×103cells/well. After attachment, cells are treated with different doses of metformin/compound C for 0 min, 15 min, 1 h, and 24 h. MTT assays are performed as described previously. In brief, MTT (5 mg/mL) is added to the 96-well plates at a volume of 10 μL/well, and the plates are incubated for 4 h. The MTT reaction is terminated by removal of the culture medium containing MTT, and 100 μL DMSO per well are added and incubated at RT on a shaker for 10 min to ensure that the crystals had dissolved sufficiently. Absorbance values are measured at 595 nm. Cell proliferation (percentage of control) is calculated as follows: absorbance (experimental group)/absorbance (control group). Cell proliferation inhibition (percentage of control) is calculated as follows: 100%−cell proliferation (percentage of control). Each experiment is performed in duplicate and repeated six times to assess result consistency. |
| Animal Admin | The animals are randomized into six groups consisting of six rats each. Rats in group 1 (control) receives a subcutaneous injection of physiological saline (0.5 mL) and are left untreated for the entire experimental period. Rats in group 2 receives an oral administration of metformin (100 mg/kg; twice daily) for 2 days and are subcutaneously injected with saline at an interval of 24 h for 2 consecutive days. Rats in group 3 (MI control) receives an oral administration of saline (twice daily) for 2 days and are sc injected with isoproterenol (100 mg/kg) daily for 2 consecutive days at an interval of 24 h. Rats in groups 4 to 6 are treated with metformin at 25, 50, and 100 mg/kg. Metformin is dissolved in saline and is gavaged at a volume of 0.25-0.5 mL twice a day at an interval of 12 h, started immediately before isoproterenol injection. |
| References |
| Boiling Point | 224.1ºC at 760 mmHg |
|---|---|
| Melting Point | 223-226 °C(lit.) |
| Molecular Formula | C4H12ClN5 |
| Molecular Weight | 165.625 |
| Flash Point | 89.3ºC |
| Exact Mass | 165.078125 |
| PSA | 88.99000 |
| LogP | 1.05850 |
| Vapour Pressure | 0.0929mmHg at 25°C |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R22;R36/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DU1800000 |
| HS Code | 2925290090 |
|
~77% 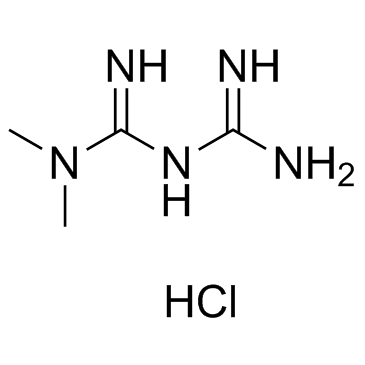
1115-70-4 |
| Literature: HANALL PHARMACEUTICAL COMPANY. LTD Patent: WO2008/93984 A1, 2008 ; Location in patent: Page/Page column 29 ; |
|
~94% 
1115-70-4 |
| Literature: Kelarev, V. I.; Bellul', M.; Zav'yalov, V. I.; Ammar Dibi; Golovin, A. N.; et al. Journal of Organic Chemistry USSR (English Translation), 1988 , vol. 24, # 5 p. 994 - 998 Zhurnal Organicheskoi Khimii, 1988 , vol. 24, # 5 p. 1100 - 1105 |
|
~% 
1115-70-4 |
| Literature: Pharmaceutical Chemistry Journal, , vol. 21, # 12 p. 892 - 894 Khimiko-Farmatsevticheskii Zhurnal, , vol. 21, # 12 p. 1517 - 1518 |
| Precursor 3 | |
|---|---|
| DownStream 4 | |
| HS Code | 2925290090 |
|---|---|
| Summary | 2925290090 other imines and their derivatives; salts thereof。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |